Identification of Antioxidant Compounds in Fig Leaves (Ficus carica L) Fractions Using LC-MS/MS
by Rahmatul Qodriah ★ , Shirly Kumala, Syamsudin Syamsudin, Nancy Dewi Yuliana, Partomuan Simanjuntak, Ervina Putri
Academic editor: Muhammad Sulaiman Zubair
Sciences of Pharmacy 2(4): 225-231 (2023); https://doi.org/10.58920/sciphar02030012
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
13 Sep 2023
06 Nov 2023
09 Nov 2023
09 Nov 2023
Abstract: The fig plant (Ficus carica L.) is one of the most popular ficus genera and is spread in tropical and sub-tropical regions throughout the world. Fig leaves have potential as antioxidants. The purpose of this study was to identify antioxidant compounds in the ethanol extract of fig leaves of the Iraqi variety. The research involved preparing extracts by maceration using 96% ethanol, 70% ethanol, and 50% ethanol, testing antioxidant activity and identifying their chemical structures using LC-MS/MS. Based on the results of the antioxidant activity test using the DPPH free radical scavenging method, it was shown that the 50% ethanol extract of fig leaves had a very strong antioxidant activity with an IC50 value of 23.36 µg/mL. The results of identification with LC-MS/MS, it is estimated that the compound that has the potential as an antioxidant in the fig leaves of the Iraqi variety is the kaempferol compound.
Keywords: Fig leavesFicus carica LAntioxidantDPPHLC-MS-MS
Introduction
In everyday life, our bodies are often exposed to harmful substances which can come from air pollution, ultraviolet light, cigarette smoke, fast food and so on. These substances can play a role in the oxidation process in the bod (1). These reactions can produce free radical compounds that can damage the structure and function of cells. Free radicals are molecules that contain one or more unpaired electrons in their outer orbitals. Unpaired electrons can cause these compounds to become very reactive in looking for partners by attacking or binding to electrons in surrounding molecules such as lipids, proteins or DNA (2). As a person ages, the possibility of the body being exposed to pollutants will be higher. This can cause the body's cells to degenerate, disrupt metabolic processes, and decrease the immune response. These factors can trigger the emergence of various degenerative diseases (3). Therefore, the human body needs an important substance, such as an antioxidant that can slow down or prevent damage caused by free radicals by reducing free radical activity or breaking the chain of oxidation reactions caused by free radicals (4).
The majority of goods classified as herbal and traditional plant remedies are also derived from extracted phytochemicals or dietary plants high in antioxidants. In our analysis of different diets, herbal and traditional plant medicines turned out to be several of the highest antioxidant-containing goods (5). Antioxidant activity testing on leaves was also carried out in Eva Agustina's research (2017), methanol extract of fig leaves has higher antioxidant activity than water and methanol-water extracts of fig leaves with an IC50 value of 32,05 µg/mL (6). The most commonly used solvents in extracting fig leaves are methanol and ethanol. However, the use of methanol as a solvent is not so recommended, because it is less safe when consumed by humans (7).
Therefore, in this study, 96% ethanol, 70% ethanol and 50% ethanol will be used as solvents to extract fig leaves (Ficus carica L.) by maceration which is then tested for antioxidant activity using the DPPH free radical scavenging method on the three extracts. The most active extract from fig leaves was partitioned using water - ethyl acetate (1:1), then the results of ethyl acetate and water partitions were tested for antioxidant activity using the DPPH free radical scavenging method. The most active partition results will be analyzed by TLC method and column chromatography. Then the fractions obtained were tested for antioxidant activity to obtain the fraction that had the highest antioxidant activity which was then identified by LC-MS / MS method so that the chemical structure was known.
Experimental Section
Materials
Fig leaves (Ficus carica L.) obtained from the Khazanah fig garden in the Cikarang area, West Java. DPPH (1,1-Diphenyl-2-Pikrilhidazyl) powder, 96% ethanol, 70% ethanol, 50% ethanol, pro-analytical methanol, n-hexane, ethyl acetate, dichloromethane, were purchased from Sigma Chemical Company (Sigma Aldrich, Singapore), vitamin C (INFALABS-BPOM). The main equipment used consisted of a UV-Vis Spectrophotometer (Shimadzu), LC-MS/MS (LC system: ACQUITY UPLC®, MS: Xevo G2-S QTof), GF254 silica gel plates, 254 nm ultraviolet lamp, rotavapor (Stuart and Eyela), micropipette (Transferpette), and other glassware.
Plant Determination
The determination of this plant was carried out at the Indonesian Institute of Sciences (Plant Conservation Research Center and Botanical Gardens), Bogor with letter number B-481/IV/D1.01/4/2021.
Preparation of Extracts
Extraction of fig leaves simplicia was carried out by maceration method using 96% ethanol, 70% ethanol and 50% ethanol 500 mL each 6 times, then the macerate was collected and concentrated using a rotary evaporator vacuum until it thickened. Each extract underwent antioxidant activity testing using the DPPH method detailed in section 2.7.
Partition of Extract with Highest Antioxidant Activity
Partitioning was carried out on the ethanol extract of fig leaves which had the highest antioxidant activity using ethyl acetate – water solvent, then shaken gently to form two layers of ethyl acetate phase and aqueous phase. The water phase was partitioned again with ethyl acetate solvent, repeated in the same way until the ethyl acetate solvent was colorless (no suspended matter). The top layer was the ethyl acetate phase and the bottom layer was the water phase. The two phases were collected, then concentrated using a rotary vacuum evaporator. Partitioning aims to separate compounds based on their polarity so that it affects the compounds contained in each fraction. The ethyl acetate and water phases were tested for their antioxidant activity using the DPPH free radical scavenging method.
Thin Layer and Column Chromatography
Partition phase with highest antioxidant activity (ethyl acetate phase) was spotted on a silica gel GF254 plate to determine the optimal mobile phase. The mobile phase used was n-hexane – ethyl acetate with a saturated ratio of 10:1, 5:1 and 2:1. Column chromatography was then carried out with the optimum mobile phase obtained from the TLC process, starting from the mobile phase n-hexane and ethyl acetate with a ratio of 10:1, 8:1, 6:1, 4:1, 2:1 and 1:1. Each fractions underwent antioxidant activity testing using the DPPH method.
Identification of Chemical Structures Using the LC-MS/MS Method
Fraction with the highest antioxidant activity (fraction VIII) of fig leaves was dissolved in pro-analyzed methanol, filtered with a syringe filter and injected into the LC-MS/MS apparatus. After the retention time is complete, the molecular weight and molecular fragmentation pattern of the compound can be determined.
Antioxidant Activity
Methanol was added to 5.0 ml to create a solution of 1.0 ml of 0.4 mmol DPPH. The resulting DPPH sample was then allowed to sit at room temperature for 30 minutes in a dark lab closet. Next, a UV-Vis spectrophotometer with a wavelength of 400–600 nm was used to detect the absorption.
Antioxidant activity testing with percent inhibition in both extracts, partitions, and fractionations used a concentration of 40ppm, each test tube and positive control received 1 mL of DPPH solution (0.4 mM), followed by the addition of 5.0 mL of methanol before analysis and homogenization. After 30 minutes of incubation, the maximum absorption wavelength was used to measure the absorption using UV-Vis spectrophotometry. Finding the IC50 value and percent inhibition Equation 1 is used to calculate the percent inhibition.
Standard curve was made between the inhibition percentage and the test concentration to determine the IC50 value. The IC50 value is the concentration obtained when the % inhibition is 50 from the equation y = a + bx. When the percent inhibition equals 50, the formula for calculating the IC50 value becomes 50 = a + bx.
Equation 1
Table 1. Yield of fig leaves extracts.
|
No. |
Solvent |
Simplicial powder (g) |
Thick extract (g) |
DER-native (g) |
Rendemen (%) |
|
1 |
Ethanol 96% |
300 |
28.41 |
10.56 |
9.47 |
|
2 |
Ethanol 70% |
300 |
27.83 |
10.78 |
9.28 |
|
3 |
Ethanol 50% |
300 |
22.60 |
13.27 |
7.53 |
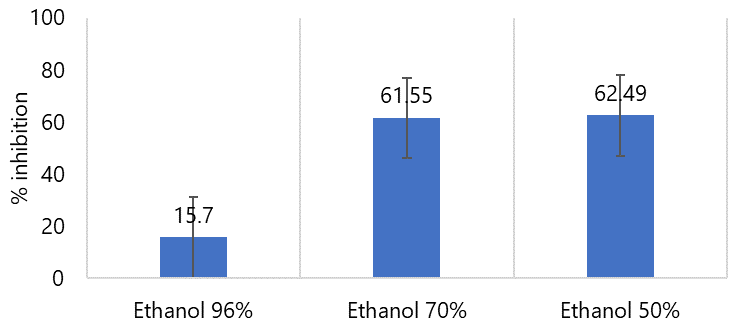
Figure 1. Percent inhibition value of antioxidant activity test of 96%, 70, and 50% fig leaves ethanol extracts.
Result and Discussion
Extraction of Fig Leaves Simplisia
Approximately 300 grams of fig leaves simplicia powder was extracted by maceration each using 96% ethanol, 70% ethanol and 50% ethanol 6 times with 500 mL of each solvent. The maserate was filtered, collected and concentrated using a rotary vacuum evaporator to obtain a thick extract. Ethanol is the solvent most often used for extraction because it is environmentally friendly, relatively safe for human health and universal. The difference in ethanol concentration can also affect the extraction results, because it results in a change in the polarity of the ethanol so that it can affect the solubility of the compounds contained in a sample.
In Table 1, the yield of 96% ethanol extract of fig leaves shows that 96% ethanol solvent is a universal solvent that can dissolve various kinds of compounds contained in both polar and semi-polar samples so that they can dissolve compounds better. In line with Nurhayati et al. (2009), that a high yield value indicates the number of bioactive components contained in it (8).
Antioxidant Activity Test of Fig Leaves Extracts
This test aims to select one of the 96, 70 and 50% ethanol extracts of fig leaves that has the highest antioxidant activity using the DPPH free radical scavenging method. Antioxidant activity testing was carried out by measuring the maximum absorption wavelength at 515.5 nm, incubation time for 30 minutes and at one concentration 40 ppm related to the effectiveness of research time. Comparison diagram of percent inhibition value of antioxidant activity test on 96%, 70%, and 50% can be seen in Figure 1.
The percent inhibition value of the 50% ethanol extract is larger than the other two solvents. The result indicated that the compounds with high polarity might be dominant in the fig and could be potentially extracted using a polar solvent (9).
Partition of Extract with Highest Antioxidant Activity
Partitioning from 50% ethanol extract aims to separate compounds based on their polarity so that it affects the compounds contained in each fraction. The different profiles of these compounds may affect their antioxidant activity. Based on the partitioning results, the ethyl acetate fraction and water fraction were 2.03 grams and 13.25 grams with yield values of 0.68% and 4.41%. Partition with ethyl acetate gets a higher yield value, meaning that the nature of secondary metabolite compounds in this section is similar to ethyl acetate in terms of polarity (8).
Antioxidant Activity Fig Leaves Partitions
As shown in Figure 2, the ethyl acetate phase to select one has the highest antioxidant activity using the DPPH free radical scavenging method. This can be caused because fig leaves contain flavonoids that are semipolar so that they dissolve in ethyl acetate which is also semipolar. Flavonoids have potential as antioxidants because they can donate hydrogen from their hydroxyl groups to free radicals (10).
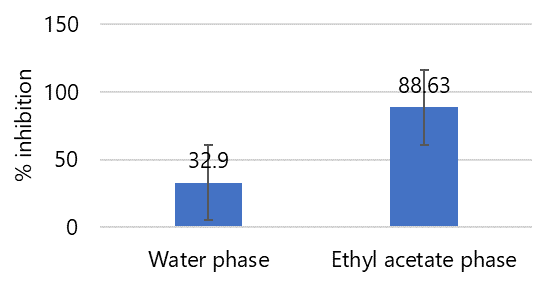
Figure 2. Percent inhibition value of antioxidant activity test of ethyl acetate phase and water phase of 50% ethanol extract of fig leaves.
Fractionation of Ethyl Acetate Phase by Column Chromatography
A total of approximately 2.03gram of ethyl acetate phase of fig leaves was fractionated by column chromatography. The purpose of fractionation is to obtain simpler compounds before structural identification by liquid chromatography-mass spectrometry. The stationary phase used was silica gel GF254 and the mobile phase used was n-hexane - ethyl acetate in the ratio (10:1), (8:1), (6:1), (4:1), (2:1), and (1:1). Purification by column chromatography is carried out in a gradient manner where the composition of n-hexane - ethyl acetate changes gradually to be able to separate the chemical compounds in the ethyl acetate phase of fig leaves properly. Fractionation of the ethyl acetate phase of fig leaves with column chromatography obtained 119 fractions, then the fractions were re-analyzed using TLC. Fractions with the same chromatogram pattern were combined to obtain 8 combined fractions. The results of the fractionation of the ethyl acetate phase of fig leaves by column chromatography can be seen in Table 2 and the TLC results of fractions 1 to 8 can be seen in Figure 3.
Table 2. Fractionation of ethyl acetate phase by column cormatography.
|
No. |
Fraction |
Bottle |
Fraction Weight (g) |
|
1 |
I |
1-11 |
0.05 |
|
2 |
II |
12-17 |
0.09 |
|
3 |
III |
18-37 |
0.17 |
|
4 |
IV |
38-44 |
0.04 |
|
5 |
V |
45-63 |
0.11 |
|
6 |
VI |
64-89 |
0.17 |
|
7 |
VII |
90-102 |
0.08 |
|
8 |
VIII |
107-119 |
0.09 |
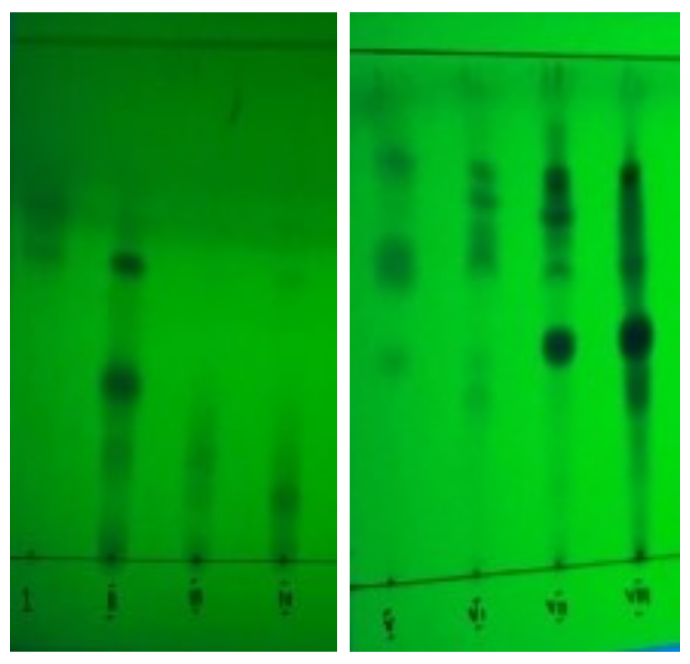
Figure 3. Fractions of ethyl acetate phase under UV light at 254 nm wavelength.
Antioxidant Activity of Fig Leaves Fractions
The stationary phase used is silica gel GF254 and the mobile phase used is n-hexan - ethyl acetate with a ratio of (10:1), (8:1), (6:1), (4:1), (2:1), and (1:1). Purification by column chromatography is carried out in a gradient manner where the composition of n-hexane - ethyl acetate changes gradually to be able to separate the chemical compounds in the ethyl acetate phase of fig leaves properly. Fractionation of the ethyl acetate phase of fig leaves with column chromatography obtained 119 fractions, then the fractions were re-analyzed using KLT. Fractions with the same chromatogram pattern were combined so that 8 combined fractions were obtained
Antioxidant activity testing of eight combined fractions aims to select one of the combined fractions that has the highest antioxidant activity where the fraction will be used to determine the IC50 value. The results of antioxidant activity testing with DPPH free radical scavenging method against 8 combined fractions can be seen in Figure 4.
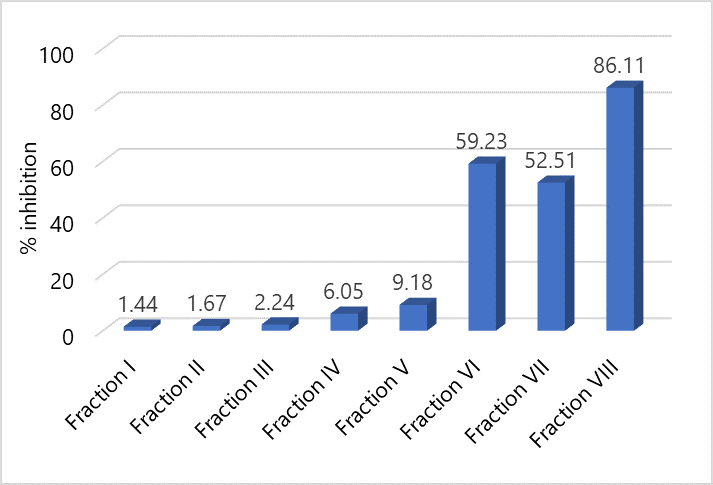
Figure 4. Graph of Comparison of Percent Value of Inhibition of Antioxidant Activity Test of the Fraction 1 - 8 of Fig Leaves at a Concentration of 40 ppm.
Based on the results of the antioxidant activity test using the DPPH free radical scavenging method, it was shown that the combined fraction VIII had a DPPH free radical scavenging activity of 86.12% which was higher than the other fractions. Based on this, the determination of the IC50 value will be continued by using the VIII fraction.
Antioxidant Activity Test on Vitamin C and Fraction VIII of Fig Leaves
The antioxidant activity test for the antioxidant compound vitamin C as a positive control was used to compare its antioxidant activity with fraction VIII as a sample. Standard curve for both vitamin C and fraction VIII can be seen in Figure 5.
The IC50 values for vitamin C and fraction VIII from fig leaves were 4.28 µg/mL and 23.36 µg/mL, respectively, indicating strong antioxidant activity (IC50 < 50 µg/mL). According to Jun et al. (2003), antioxidant strength is classified as strong (IC50 < 50 ppm), active (IC50 50-100 ppm), moderate (IC50 101-250 ppm), weak (IC50 250-500 ppm), and inactive (IC50 > 500 ppm) (11).
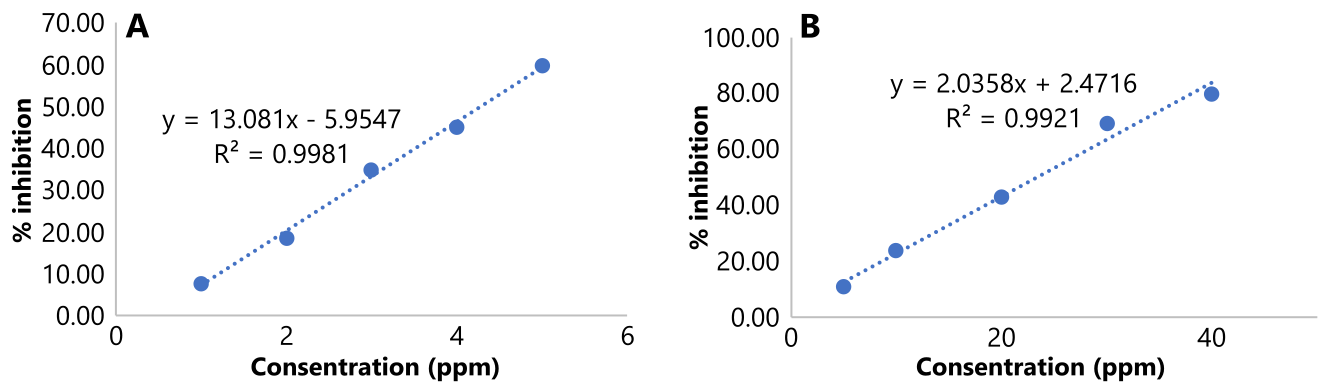
Figure 5. Standard curve of (A) ascorbic acid and (B) fraction VIII of fig leaves in DPPH antioxidant test.
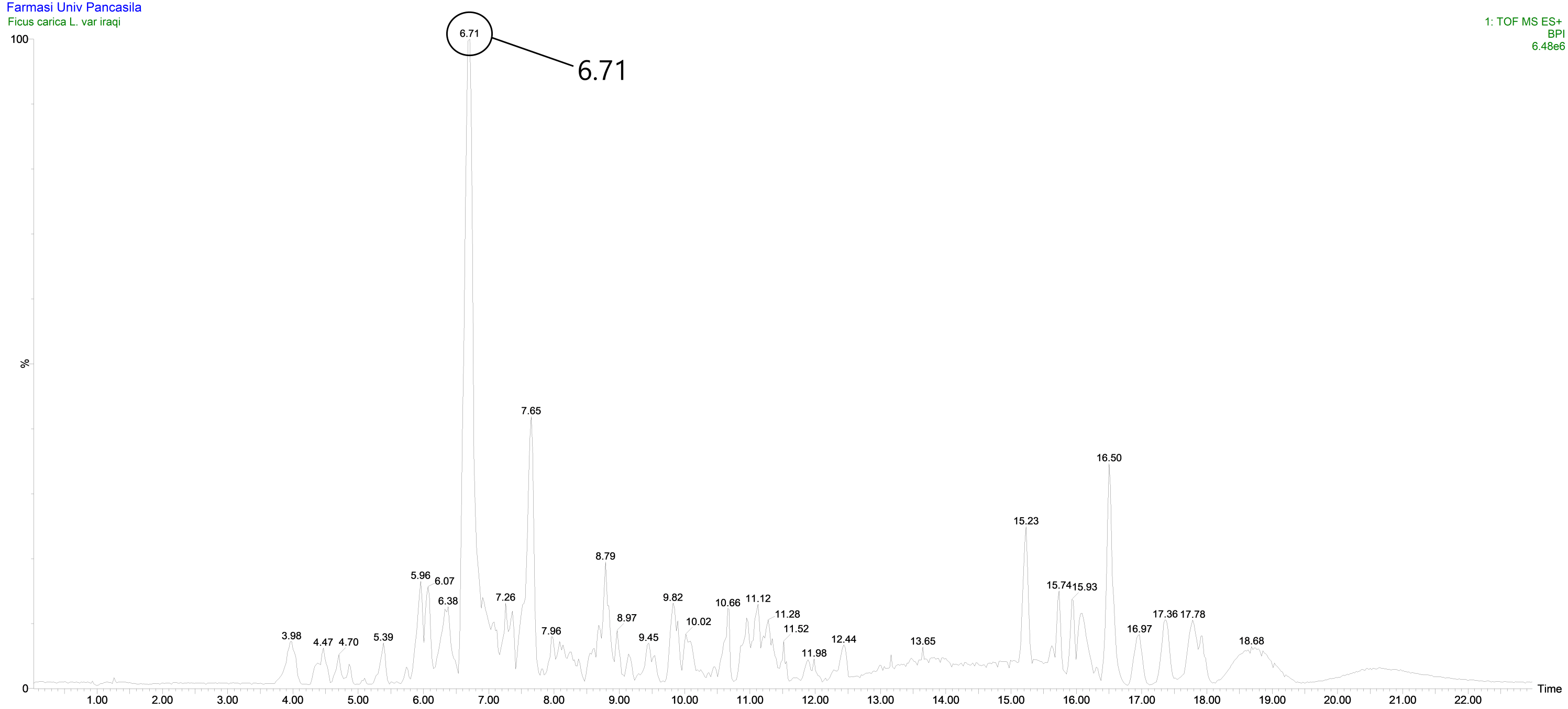
Figure 6. Kromatogram LC-MS/MS fraction VIII fig leaves.
Identification of Chemical Structures with the LC-MS/MS Method
The number of peaks produced indicated that the compounds in fraction VIII were not yet pure, but there was one peak that was identified with a retention time of 6.71 minutes and had an intensity or relative abundance of 100% so that it was declared a base peak. Based on the results of data analysis using Masslynk software and the Massbank database, the molecular formula C15H10O6 is obtained with a molecular mass of [M+H]+ m/z = 287.0560. This shows that the fig leaves fraction VIII has a molecular mass of [M] m/z = 286.0560.
Based on the results of the analysis above, kaempferol and luteolin were found to share the same molecular formula and molecular weight, leading to the need for a re-identification process by comparing the fragmentation of fraction VIII of fig leaves with existing literature. Research conducted by Raymond E. March and Xiu-Sheng Miao (2004) revealed that kaempferol had a molecular weight of [M+H]+ m/z = 287 (12) and a daughter ion equation similar to that of the VIII fraction of fig leaves. In the retention time of 6.71 minutes, fraction VIII of fig leaves exhibited parent ions at [M+H]+ m/z = 287.0560 and several daughter ions [M+H]+ m/z = 121.0293; m/z = 148.1132; m/z = 152.0195; m/z = 165.0192; and m/z = 213.0509 (refer to Figure 7). Filip Cuyckens (2004) reported a comparison between the fragmentation of kaempferol and luteolin, and the fragmentation of fraction VIII of fig leaves was found to be similar to the fragmentation of kaempferol (12).
Based on the results of the analysis above, it was determined that the active compounds that had activity as antioxidants in fig leaves were thought to be kaempferol compounds. Kaempferol was considered to be an antioxidant compound in fig leaves. This align with the research of Zhongyuan Li et al. (2021), where fig leaves were found to contain kaempferol compounds (13).
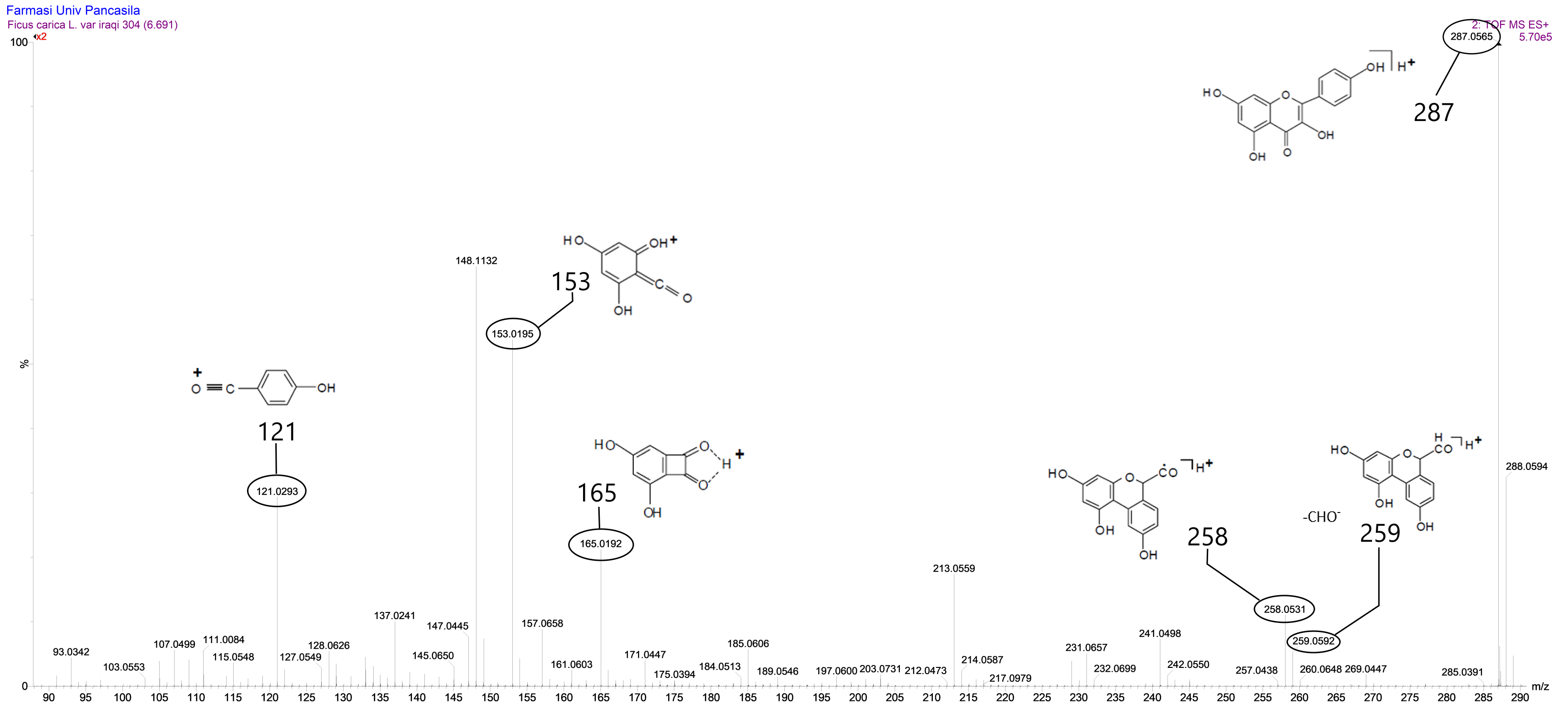
Figure 7. Mass spectrum of fig leaves fraction VIII at retention time of 6.71 minutes.
Kaempferol is a flavonoid compound in the flavonol class which has many benefits, one of which is as an antioxidant. This was confirmed by the presence of flavonoid compounds in the preliminary phytochemical screening test (11). The mechanism of action of flavonoids as antioxidants can be directly by donating hydrogen ions so that they can neutralize the toxic effects of free radicals and indirectly by increasing the expression of endogenous antioxidant genes through activation of Nuclear factor erythroid 2 related factor 2 (Nrf2) resulting in an increase in genes that play a role in the synthesis of endogenous antioxidant enzymes such as the SOD (Superoxide dismutase) gene (14-16).
Conclusion
The fig simplicia (Ficus carica L.) var. Iraqi contained secondary metabolites, including flavonoids, saponins, triterpenoids, and tannins. The most potent antioxidant within the extracts was identified in the 50% ethanol extract of fig leaves (Ficus carica L.) var. Iraqi, with an IC50 value of 23.36 µg/mL. Based on the results of identification using LC-MS/MS, it was determined that the compound acting as an antioxidant was the kaempferol compound, which belonged to the flavonol class of flavonoid compounds.
Declarations
Acknowledgment
The authors thank the support of the faculty of pharmacy, Pancasila University, South Java, Indonesia.
Ethics Statement
Not applicable.
Data Availability
All data supporting the findings of this study are available within the paper.
Funding Information
This research received internal funding from the faculty of pharmacy, Pancasila University, South Java, Indonesia, with grant number of 007/FF-UP/NPJ/XII/2022.
Conflict of Interest
The authors declare no conflicting interest.
References
- Shamkant B. Badgujar, Vainav V. Patel, Atmaram H. Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. 2014. Pharm Biol 52(11): 1487–1503
- Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB. Ficus carica L. metabolic and biological screening. Food and Chemical Toxicology. 2009; 47(11): 2844-2845
- M Ramgopal, G Muniswamy, M Balaji , Koorbanally NA , Md. Shahidul Islam. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. Journal of food and drug analysis. 2018; 26: (201-210)
- Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB. Ficus carica L.: metabolic and biological screening. Food and Chemical Toxicology. 2009; 47(11): 2844-2845
- Badgujar SB, Patel VV, Bandivdekar AH, Mahajan RT. Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharmaceutical Biology. 2014; 52(11): 1487-1488, 1491-1493
- Agustina E. Antioxidant compound activity test of tiin (Ficus carica Linn) leaves extract with water, methanol and methanol-water mixture solvents. KLOROFIL: Journal of Biological and Applied Sciences. 2017; 1(1): 39
- Li Z, Yang Y, Liu M, Zhang C, Shao J, Hou X, Tian J, Cui Q. A comprehensive review on phytochemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. leaves. Biomedicine & Pharmacotherapy. 2021; 137:111393
- Nurhayati T, Aryanti D. Nurjanah. 2009. Preliminary assessment of sponge extract potential as antioxidant. Jurnal Kelautan Nasional.2009;(2):43-51 33.
- Yarnpakdee S, Benjakul S , Senphan T. Antioxidant Activity of the Extracts from Freshwater Macroalgae (Cladophora glomerata) Grown in Northern Thailand and Its Preventive Effect against Lipid Oxidation of Refrigerated Eastern Little Tuna Slice. 2018. Turk. J. Fish.& Aquat. Sci. 19(3), 209-219.
- Jun M, Fu HY, Hong J, Wan X, Yang CS, Ho CT. Comparison of Antioxidant Activities of Isoflavones from Kudzu Root (Pueraria lobata Ohwi). J Food Sci. 2003;68(6):2117-22.
- Farag RS, Abdel-Latif MS, Abd El Baky HH, Tawfeek LS. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnology Reports. 2020; 1-36
- March RE, Miao XS. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. International Journal of Mass Spectrometry. 2015;231(2-3):157-67
- Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. Journal of Mass spectrometry. 2015 Jan;39(1):1-5.
- Li Zhongyuan, et al. A comprehensive review on phytochemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. leaves. Biomedicine & Pharmacotherapy. 2021; 137:111393
- Perez C, Canal JR, Torres MD. Experimental diabetes treated with Ficus carica extract: effect on oxidative stress parameters. Acta Diabetol 2003;40:3-8.
- Belguith-Hadriche O, Ammar S, Contreras Mdel M, Turki M, Sequra-Carretero A, Ei Feki A, Makni-Ayedi F, Bouaziz M. Antihyperlipidemic and antioxidant activates of Edible Tunisian Ficus carica L. Fruits in high fat diet induced hyperlipidemic rats. Plant Foods Hum Nutr 2016;71:183-9

 ETFLIN
Notification
ETFLIN
Notification






