
 ETFLIN
Notification
ETFLIN
Notification
Sciences of Pharmacy (SciPhar) is an international, peer-reviewed open-access journal of pharmacy published by ETFLIN. We offer a platform and place for researchers and intellectuals, especially the youth, to share their insights and works. SciPhar accepts original article, reviews, mini-review, book-review, technical note, case report, case series, clinical trial, opinion/perspective, conference proceeding, and pictorial essay. Author may submit or suggest another type of scientific manuscript. Sciphar publishes 4 issues a year. Sciences of Pharmacy is affiliated with Faculty of Pharmacy, Universitas Islam Sultan Agung.
Authors from countries other than Indonesia are not charged a publication fee until further notice.
[Prompt rejection will be given for AI generated papers]
Scope
Sciphar covers all aspects of pharmacy, including but not limited to:
Prof. Dr. Moelyono Moektiwardoyo, MS., Apt.
Department of Biology Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang 45363, Indonesia
With profound respect, we commemorate Prof. Dr. Moelyono Moektiwardoyo, MS., Apt., as Editor Emeritus (In Memoriam). His enthusiasm for valuing natural resources and utilizing them wisely will continue to inspire us all. Thank you, Prof. Moelyono, for your dedication and invaluable intellectual legacy to society and science. May he rest in eternal peace.
Prof. Yashwant V Pathak, M. Pharm., Executive MBA, MSCM, Ph.D., FAAAS
USF Health Taneja College of Pharmacy, University of South Florida, Florida 33612, USA
Expertise: Nanotechnology; Physical chemistry; Polymer chemistry; Nutraceuticals.
Scopus ID Google Scholar ResearchGate
Our esteemed editorial board comprises 36 experts from 17 different countries across 5 continents. Our board members bring a diverse range of knowledge, skills, and experiences to the table, enabling us to provide a broad perspective on various issues.
Prof. Elena Bakhrushina, Ph.D.
Departament of Pharmaceutical Technology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia
Expertise: Controlled drug delivery; Ocular drug delivery; Pharmaceutical biotechnology; Spray drying; Pharmacokinetics; Biopharmaceutics; Industrial pharmacy; Pharmaceutical analysis; Targeted drug delivery; Solid dosage forms.
Google Scholar ResearchGate Scopus ID ORCID
Prof. Dr. apt. Marline Abdassah Bratadiredja, MS
Departement of Pharmaceutic and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang 45363, Indonesia
Expertise: Transdermal drug delivery; Cosmetics; Extract formulation.
Google Scholar Scopus ID
Prof. Jittima Amie Luckanagul, Ph.D.
Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok 10330, Thailand
Expertise: Polymers synthesis; Liposomal delivery system; Vaccines; Nanoparticles.
Google Scholar Scopus ID Research Gate ORCID
Prof. Saule Kutymovna Ordabaeva, Ph.D.
Department of Pharmaceutical and Toxicological Chemistry, Faculty of Pharmacy, South Kazakhstan Medical Academy, Shymkent 160001, Kazakhstan
Expertise: Pharmaceutical production technology; Pharmaceutical ecology.
Prof. Dr. V Vidyashree Nandini, MDS, DNB, MAMS
SRM Kattankulathur Dental College and Hospital, SRMIST, POTHERI-603203, Kattankulathur Campus, Kattankulathur, Tamil Nadu 603203, India
Expertise: Implant dentistry; Biomaterials; Dental materials; Prosthodontics; Esthetic dentistry; Temporomandibular disorders.
Google Scholar ResearchGate Scopus ID ORCID
Prof. apt. Muhammad Sulaiman Zubair, M.Si., Ph.D.
Natural Product Research Group, Department of Pharmacy, Faculty of Science, Tadulako University, Palu 94118, Indonesia
Expertise: Phytochemistry; Organic chemistry; Medicinal chemistry.
ORCID Google Scholar Scopus ID Research Gate
Dr. apt. Rina Wijayanti, M. Sc.
Department of Pharmaceutical Sciences, Faculty of Pharmacy, Universitas Islam Sultan Agung, Kaligawe Raya Street Km. 4, Semarang, Indonesia
Expertise: Pharmacognosy; Herbal medicine.
Scopus Google Scholar
Ahmed Mahal, Ph.D.
Department of Medical BioChemical Analysis, Faculty of Health Technology, Cihan University-Erbil, Iraq
Expertise: Medicinal Chemistry
Scopus Google Scholar ORCID
Dr. Adeleye Ademola Olutayo, PGDE
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Olabisi Onabanjo University, Ago-Iwoye, Nigeria
Expertise: Drug formulation; Controlled drug delivery; Preformulation; Drug stability; Tablet; Pharmaceutical formulation; Medical nanotechnology; Topical administration.
Google Scholar ResearchGate Scopus ID ORCID
Dr. Sayani Bhattacharyya, M.Pharm
Krupanidhi College of Pharmacy, Bengaluru, Karnataka 560035, India
Expertise: Solid state behaviour; Cocrystallization; Mesoporous materials; Oral disintegrating films.
Google Scholar ResearchGate Scopus ID ORCID
Dr. apt. Garnadi Jafar, M.Si
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Bhakti Kencana, Bandung City 40614, Indonesia
Expertise: Herbal formulation.
Google Scholar Scopus ID
Dr. Maryam Bikhof Torbati
Department of Biology, Islamic Azad University-Shahre rey Branch, Tehran 1477893855, Iran
Expertise: Cancer nano drug delivery systems; Molecular biology; Biotechnology; Cell biology; Medicinal chemistry.
Google Scholar ResearchGate Scopus ID ORCID
Burak Kuzu, Ph.D.
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Van Yüzüncü Yıl University, 65090 Tuşba/Van, Turkey
Expertise: Medicinal chemistry; Organometallic Chemistry; Organic Chemistry.
Google Scholar ResearchGate
Dr. Ebrahim Tavakoli
Department of Biomedical Engineering, Faculty of Engineering, University of Isfahan, Isfahan, P.O. Box 8174613441, Iran
Expertise: Stem cell therapy; Tissue replacement via advanced materials; and Drug delivery nano-carrier systems.
ResearchGate
Dr. Abdelrahman I. Rezk
Department of Bionanosystem Engineering, Graduate School, Chonbuk National University, Jeonju-Si 561-756, Republic of Korea
Expertise: Biomedical devices; Nanomedicine
Google Scholar Scopus ORCID
Prof. Dr. Pilli Govindaiah
Department of Pathology, School of Medicine, Wayne State University, Detroit, Michigan 48202, USA
ResearchGate Google Scholar ORCID Scopus ID
Mohd Shahezwan Abd Wahab, M.Clin.Pharm., Ph.D.
Faculty of Pharmacy, Universiti Teknologi MARA, Puncak Alam, Malaysia
Expertise: Clinical Pharmacy; Hospital Pharmacy; Pharmacy Education; Complementary & Alternative Medicine; Pharmaceutical Care; Community Pharmacy; Medication Therapy Management.
ResearchGate Google Scholar Scopus ORCID
Dr. Zilin Wei
Key Laboratory of Risk Assessment and Control for Environment & Food Safety, Tianjin Institute of Environmental & Operational Medicine, Tianjin, China
Expertise: Microbiology; Genetics; Bioinformatics.
ResearchGate Scopus ID Google Scholar
Dr. Constancy Prisca Aleru-Obogai, B.MLS., AMLSN., M.Sc.
Department of Medical Laboratory Science, Rivers State University of Science & Technology, Nkpolu-Oroworukwo, Port Harcourt, Nigeria
Expertise: Medical Microbiology; Pharmaceutical Microbiology; Diagnostic Parasitology; Medical Mycology; Clinical Bacteriology; Clinical Chemistry; Clinical Infectious Diseases; PCR; Clinical Hematology; Medical Parasitology; Clinical Microbiology; Nosocomial Infection; Diagnostic Microbiology; Antimicrobial Susceptibility Testing; Antimicrobials; Bacteriology; Antibiotic Resistance.
ORCID Google Scholar ResearchGate
Dr. Mustafa Azizoğlu, MD
Department of Stem Cell and Tissue Engineering, Istinye University, 34396 Istanbul, Turkey
Expertise: Stem cell; Tissue engineering; Minimal invasive surgery
ORCID Google Scholar Scopus
Prof. Mohammad B. Nusair, M.Sc., Ph.D.
Sociobehavioral and Administrative Pharmacy Department, Nova Southeastern University, Florida, United States
Expertise: Health Care Management; Health Services Research; Pharmaceutical Care; Mixed Methods; Quantitative and Qualitative Research; Deprescriptions; Polypharmacy; Pharmacy Education.
Google Scholar ResearchGate Scopus ID ORCID
Taehwan Park, Ph.D.
Pharmacy Administration and Public Health, College of Pharmacy and Health Sciences, St. John's University, Queens, New York 11439, United States
Expertise: Pharmacoeconomic, pharmacoepidemiology, social pharmacy, digital pharmacy, telepharmacy, big pharmacy data.
ResearchGate Scopus ID
Ruth Jeminiwa, Ph.D.
Department of Pharmacy Practice, Jefferson College of Pharmacy, Thomas Jefferson University, Philadelphia, United States
Expertise: Health outcomes research (behavioral health, digital health, pharmacoeconomics).
Google Scholar ResearchGate Scopus ID ORCID
Dr. Indriyati Hadi Sulistyaningrum, M. Sc
Department of Pharmaceutical Sciences, Faculty of Pharmacy, Universitas Islam Sultan Agung, Kaligawe Raya Street Km. 4, Semarang, Indonesia
Expertise: Clinical pharmacy; Community pharmacy
Scopus Google Scholar
Dr. dr. Cosmos Octavianus Mangunsong, SpM(K)
Indonesian Ophthalmologist Association [IOA] – PERDAMI, Jakarta, Indonesia
Expertise: Telemedicine; Ocular therapy; Stem cell therapy; Regenerative medicine for ocular diseases.
apt. Fatma Aldila, S.Farm., M.I.P.H. M.H.M (Exn.)
Department of Clinical Studies, PT. Nalagenetik Riset Indonesia, Indonesia
Expertise: Pharmacoepidemiology; Pharmacoeconomics; Digital health; Medication safety; Health workforce.
Assist. Prof. Ahmed Mohsin Mahdi, Ph.D.
Assistant Dean of the College of Computer Science and Information Technology, Al-Qadisiyah University, Iraq
Expertise: Genetic aericultural analysis; Brain EEG clustering; Machine learning.
Google Scholar Scopus
Dr. Ernest Domanaanmwi Ganaa, M.Sc.
ICT department of Hilla Limann Technical University, Ghana
Expertise: Big data in pharmacology, toxicology, and pharmaceutics; Machine learning; Pattern recognition; and Dimensionality reduction.
Google Scholar ResearchGate Scopus ID ORCID
Dr. Borra N. Dhanunjayarao, M.Tech
Vignans Institute of Information Technology, Visakhapatnam, Andhra Pradesh 530049, India
Google Scholar ResearchGate Scopus ID ORCID
Dr. Morteza Rabiei
Nanobiotechnology Department, Faculty of Innovative Science and Technology, Razi University, Kermanshah 6714414971, Iran
Expertise: Server; Distributed databases; Bioinspired engineering and biomimetic design.
Google Scholar ResearchGate ORCID
Angi Nadya Bestari, S.Farm., M.Sc., Apt.
Department of Pharmaceutics, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta 55281, Indonesia
Google Scholar
Keerthic Aswin, M.Pharm
CSIR-GATE JRF, Institute of Genomics and Integrative Biology, New Delhi, India
Google Scholar Scopus ID ResearchGate
Apt. Dhanang Prawira Nugraha., S.Farm., M.Farm
Department of Pharmacy, Faculty of Sciences and Technology, Ma Chung University, Malang 65151, Indonesia
Google Scholar
Derevnina Ekaterina Alekseevna, M.Sc.
SOLYUR pharmaceutical company, Moscow 115432, Russia
Georgy Prosvirkin, M.Sc.
Peoples' Friendship University of Russia (RUDN University), Moscow 117198, Russia
Effective 24 December 2025, VAT (11%) will be applied to the final charge of accepted articles and included in the invoice.
To maintain a clear focus on pharmaceutical sciences, Sciences of Pharmacy will no longer consider manuscripts primarily centered on complementary, alternative, or traditional medicine practices. This includes studies focused solely on herbal therapies, natural product extraction/fractionation, acupuncture, homeopathy, spiritual healing, or other non-conventional treatment modalities.
Submissions in this area will only be considered if they demonstrate advanced pharmaceutical formulation and comprehensive physicochemical or mechanistic characterization, including aspects such as drug encapsulation efficiency, release kinetics, stability profiling, thermodynamic analysis, molecular interactions, and pharmacological pathway studies supported by multiple validation approaches that align with modern pharmaceutical science and technology. Extract studies may be accepted if accompanied by a robust pharmacological evaluation.
Articles submitted prior to the policy implementation date will still be processed and will not be affected by this change.
Starting from this date, we kindly require authors to provide a graphical abstract for their article. The image must comply with the publisher’s requirements, as outlined in the Author Instructions, Section 3.7: Graphical Abstract. This file can be submitted along with the manuscript via the submission portal, which is accessible after logging in.
If the author claims any pharmacological activity of the tested sample, a corresponding study to support this claim must be conducted and included in the manuscript. If your study focuses solely on formulation characterization or optimization, please refrain from making any claims about pharmacological activity or efficacy unless they are supported by proper evidence and included in the manuscript.
Any study involving animals or humans must include ethical approval. For studies involving humans, informed consent is required, including cases related to food or cosmetic applications. Please note that we may decline articles that only describe the formulation and basic characterization of a simple preparation without a comprehensive study and in-depth discussion.
Since our accreditation by the Ministry of Education and Culture of Indonesia (KEMENDIKBUD) through the SINTA (Science and Technology Index) program, we have implemented an Article Processing Charge (APC) for authors based in Indonesia. This policy was introduced as part of our efforts to support the long-term sustainability of ETFLIN.
No article processing charges (APC) are applied to authors from countries outside Indonesia because international authors are not able to benefit from our journal’s local indexing.
For Indonesian authors, a waiver of up to 100% may be granted if you or your manuscript falls into one of the eligible categories.
The reviewer's name will automatically appear in this list after they agree to be visible as a reviewer of an article in our peer review submission system. The names are ordered by the date of their latest contribution in real time. This makes the number and order of reviewers in this list dynamic.
Prof Manisha Duseja (Google Scholar)
Analytical Chemistry Lab, Department of Chemistry, School of Physical Sciences, DIT University, Uttarakhand, Dehradun, 248009, India
Dr Chiranjit Ghosh (Google Scholar)
Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, India
Dr Heru Nurcahyo (Google Scholar)
Department of Pharmacy, Polytechnic of Harapan Bersama, Tegal, Indonesia
Dr Yuniarti Falya (Google Scholar)
Sekolah Tinggi Farmasi Muhammadiyah Cirebon, Indonesia
Dr Amelia Ramadhani Anshar (Google Scholar)
Animal Biomedical Sciences Study Program, School of Veterinary and Biomedical Sciences, IPB University, Bogor, 16680, Indonesia
Dr Supomo (Google Scholar)
Departement of Pharmaceutical Biology, Departement of Pharmacology, Departement of Pharmaceutical Technology, Sekolah Tinggi Ilmu Kesehatan Samarinda, East Kalimantan, Indonesia
Dr Chynthia Pradiftha Sari (Google Scholar)
Department of Pharmacy, Universitas Islam Indonesia, Yogyakarta, Indonesia
Dr Tri Bayu Purnama (Google Scholar)
Faculty of Public Health, Universitas Islam Negeri Sumatera Utara, Medan, Indonesia
Prof Oguz Karabay (Google Scholar)
Department of Infectious Diseases and Clinical Microbiology, Faculty of Medicine, Sakarya University, Sakarya, Turkey
Prof Muhammad Arba (Google Scholar)
Department of Pharmaceutical Analysis and Medicinal Chemistry, Faculty of Pharmacy, Universitas Halu Oleo, Kendari, Indonesia
Dr Asadullah Madni (Google Scholar)
Department of Pharmaceutics, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan
Dr Ali Bakhshi (Google Scholar)
Nanotechnology Department, School of Advanced Technologies, Iran University of Science and Technology (IUST), Tehran, 1684613114, Iran
Dr Vincent Laiman (Google Scholar)
Department of Radiology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada – Dr. Sardjito Hospital, Yogyakarta, Indonesia
Prof Balaji P. (Google Scholar)
Department of Pharmacology, School of Pharmaceutical Sciences, Vels Institute of Science, Technology and Advanced Studies, Pallavaram, Chennai, 600 117, India
Dr Alfian Nur Rosyid (Google Scholar)
Department of Pulmonology and Respiratory Medicine, Universitas Airlangga Hospital, Surabaya, Indonesia
Dr I Nyoman Wijaya (Google Scholar)
Faculty of Pharmacy Universitas Airlangga Indonesia
Dr Dinda M. N. Ratri (Google Scholar)
Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, East Java, Surabaya, Indonesia
Dr Hikmawan Wahyu Sulistomo (Google Scholar)
Department of Pharmacology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
Dr Dharmalingam Kirubakaran (Google Scholar)
Department of Pharmacology, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Tamil Nadu, Chennai, 602105, India
Dr Paula Mariana Kustiawan (Google Scholar)
Faculty of Pharmacy, Universitas Muhammadiyah Kalimantan Timur, East Kalimantan, Samarinda, 75124, Indonesia
Dr Ushasi Das (Google Scholar)
Department of Pharmaceutical Technology, Jadavpur University, Jadavpur, West Bengal, Kolkata, 700032, India
Dr Risa Ahdyani (Google Scholar)
Department of Pharmaceutics, Faculty of Pharmacy, Universitas Muhammadiyah Banjarmasin, Gubernur Syarkawi, Barito Kuala, Kalimantan Selatan, Indonesia
Dr Faruk Jayanto Kelutur (Google Scholar)
Department of Chemistry, Pattimura University, Ambon, Indonesia
Dr Masood Soltanipur (Google Scholar)
Integrative Oncology Department, Breast Cancer Research Center, Motamed Cancer Institute, ACECR, Tehran, Iran
Dr Zulfan Zazuli (Google Scholar)
Department of Pharmacology—Clinical Pharmacy, Institut Teknologi Bandung, Bandung, 40132, Indonesia
Dr Ungky A. Setyawan (Google Scholar)
Department of Pulmonology and Respiratory Medicine, Universitas Brawijaya, Saiful Anwar General Hospital, East Java, Indonesia
Dr Samirah (Google Scholar)
Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, East Java, Surabaya, Indonesia
Dr Putu Nita Cahyawati (Google Scholar)
Faculty of Medicine and Health Science, Universitas Warmadewa, Denpasar, 80239, Indonesia
Dr Mukarram Mudjahid (Google Scholar)
Department of Pharmacy, Faculty of Pharmacy, Hasanuddin University, Makassar, Indonesia
Dr Erlia Anggrainy Sianipar (Google Scholar)
Department of Pharmacy, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia, DKI Jakarta, North Jakarta, 14440, Indonesia
Dr Ari Yuniarto (Google Scholar)
Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Muhammadiyah A.R. Fachruddin University, Tangerang, Indonesia
Prof Hayun Hayun (Google Scholar)
Faculty of Pharmacy, Universitas Indonesia, West Java, Depok, 16424, Indonesia
Prof Magdy Mostafa Desoky Mohammed (Google Scholar)
Pharmacognosy Department, Pharmaceutical and Drug Industries Research Institute, National Research Centre, Dokki, Giza, 12622, Egypt
Dr Mah Noor (Google Scholar)
Department of Microbiology and MolecularGenetics, The Women University Multan, Pakistan.
Dr Muhammad Asif (Google Scholar)
National Institute of Food Science and Technology, University of Agriculture Faisalabad, Faisalabad, Pakistan
Prof Sahar Y. Al-Okbi (Google Scholar)
Nutrition and Food Sciences Department, National Research Centre, Cairo, Egypt
Dr Archana S. Patil (Google Scholar)
Department of Pharmaceutics, KLE College of Pharmacy Belagavi, KLE Academy of Higher Education and Research, Karnataka, Belagavi, 590010, India
Prof Bhupendra Prajapati (Google Scholar)
Shree S. K. Patel College of Pharmaceutical Education and Research, Ganpat University, Mehsana, 384012, India
Dr Muhammad Iqhrammullah (Google Scholar)
Postgraduate Program of Public Health, Universitas Muhammadiyah Aceh, Banda Aceh, Indonesia
Dr Sweta Patel (Google Scholar)
Department of Pharmaceutical Quality Assurance, Parul Institute of Pharmacy and Research, Parul University, Vadodara, Gujarat, Limda, 391760, India
Dr Rolan Rusli (Google Scholar)
Mulawarman UniversityFaculty of Pharmacy
Dr Madhusree Kuanr (Google Scholar)
Department of Computer Science and Engineering, IIIT Bhubaneswar, Odisha, 751003, India
Prof. Manoj Kumar (Google Scholar)
NIPER, SAS Nagar, Punjab 160 062, India
Dr Heba A. S. El-Nashar (Google Scholar)
Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Abbassia, Cairo, 11566, Egypt
Dr Nur Fathonah Sadek (Google Scholar)
Food Technology Department, Faculty of Engineering, Bina Nusantara University, Jakarta, 11480, Indonesia
Dr Farid Dabaghian (Google Scholar)
Department of Pharmacognosy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Dr Ghader Mohammadnezhad (Google Scholar)
Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Dr Tunggul Adi Purwonugroho (Google Scholar)
Department of Pharmacy, Faculty of Health Sciences, University of Jenderal Soedirman, Purwokerto, Indonesia
Dr Purbowatiningrum Ria Sarjono (Google Scholar)
Chemistry Department, Faculty of Science and Mathematics, Diponegoro University, Semarang, Indonesia
Dr Cyntiya Rahmawati (Google Scholar)
Department of Pharmacy, Muhammadiyah University of Mataram, West Nusa Tenggara, Indonesia
Prof Andi Hermansyah (Google Scholar)
Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
Dr Shahid Ali (Google Scholar)
Department of Pharmacology, University of Karachi, Pakistan
Dr Patonah Hasimun (Google Scholar)
Pharmacology and Clinical Pharmacy Research Group, Faculty of Pharmacy, Bhakti Kencana University, Bandung, Indonesia
Dr Sankha Bhattacharya (Google Scholar)
School of Pharmacy & Technology Management, SVKM’S NMIMS Deemed-to-be University, Maharashtra, Shirpur, India
Dr Anjaneyulu Vinukonda (Google Scholar)
Formulation and Process Development, Strides Pharma Inc, Chestnut Ridge, NY, United States
Dr Imtiyaz Ansari (Google Scholar)
Department of Pharmacology, Oriental College of Pharmacy, Sanpada, Maharashtra, Navi Mumbai, 400 705, India
Prof Muhammad Asif Nawaz (Google Scholar)
Department of Biotechnology Shaheed Benazir Bhutto University, Sheringal, Dir (Upper), KPK-Pakistan
Dr Mrinal Sharma (Google Scholar)
University Institute of Pharma Sciences, Chandigarh University, Gharuan, Punjab, Mohali, 140143, India
Dr Abubakar Babangida Usman (Google Scholar)
Department of Immunology, School of Medical Laboratory Sciences, Usmanu Danfodiyo University Sokoto, Sokoto, Nigeria
Dr Omneya M. Helmy (Google Scholar)
Department of Microbiology and Immunology, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Dr Putu Austin Widyasari Wijaya (Google Scholar)
Department of Physiology and Biochemistry, Faculty of Medicine and Health Science, Warmadewa University, Denpasar, Indonesia
Dr Lakshmi (Google Scholar)
KIET School of Pharmacy, KIET Group of Institutions, Ghaziabad, India
Dr Nur Rasdianah (Google Scholar)
Pharmacy Study Program, Faculty of Health and Sports, Universitas Negeri Gorontalo, Gorontalo 96128, Indonesia
Dr Raeed Altaee (Google Scholar)
Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Kerbala, Kerbala, Iraq
Prof Ivo Sirakov (Google Scholar)
Department of Medical Microbiology, Faculty of Medicine, Medical University, Sofia, 2 Zdrave, Str.,1431 Sofia, Bulgaria
Dr Ita Zuraida (Google Scholar)
Study Program of Fishery Products Technology, Department of Aquaculture, Faculty of Fisheries and Marine Science, Mulawarman University, Kota Samarinda, Indonesia
Dr Aida Sefidani Forough (Google Scholar)
Department of Pharmaceutical Care, Amiral Momenin Hospital, Zanjan University of Medical Sciences, Khodabandeh, Zanjan, Iran,
Dr Jahanzeb Kamal Khan (Google Scholar)
College of Physicians and Surgeons, Karachi, Pakistan
Prof Deniz Karataş (Google Scholar)
Manisa Celal Bayar University, Faculty of Engineering and Natural Sciences, Department of Bioengineering, 45140, Yunusemre, Manisa, Türkiye
Prof Abbas F. Almulla (Google Scholar)
Medical Laboratory Technology Department, College of Medical Technology, TheIslamic University, Najaf, Iraq.
Dr Andika Dwi Mahendra (Google Scholar)
Department of Pharmacy, dr Soeradji Tirtonegoro General Hospital Medical Center, Klaten, Indonesia
Dr Muhammad Fauzi (Google Scholar)
Faculty of Pharmacy, Islamic University of Kalimantan Muhammad Arsyad Al Banjari, Banjarmasin 70123, South Kalimantan, Indonesia
Dr Ine suharyani (Google Scholar)
Sekolah Tinggi Farmasi Muhammadiyah, Cirebon, Indonesia
Dr P S. Sujatha (Google Scholar)
Research Department of Zoology, Government Arts College (Autonomous), Coimbatore, Tamil Nadu, India
Dr Abrar Ghaith (Google Scholar)
Pharmacoeconomics, Department of Economics, Faculty of Economics and Business, Debreceni Egyetem, DEBRECEN, Hajdú-Bihar, Hungary
Prof Ramdas Bhat (Google Scholar)
Associate Professor, Department of Pharmacology, Srinivas College of Pharmacy, Valachil, Farangipete Post, Mangalore, Karnataka, India
Dr Aliasgar Shahiwala (Google Scholar)
Department of Pharmaceutical Sciences, Dubai Pharmacy College for Girls, Dubai, United Arab Emirates
Dr Mahanem Mat Noor (Google Scholar)
Centre for Biotechnology and Functional Food, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi 43600, Malaysia
Dr Ha Cam Anh (Google Scholar)
Ho Chi Minh City University of Technology, Vietnam
Prof Maria Laura Santarelli (Google Scholar)
Department of Chemical Engineering Materials and Environment, Sapienza University of Rome, Via Eudossiana 18, 00184 Rome, Italy
Dr Tantri L. Nareswari (Google Scholar)
Department of Pharmacy, Faculty of Science, Institut Teknologi Sumatera, Lampung, Indonesia
Dr Ihor Ustymenko (Google Scholar)
National University of Life and Environmental Sciences of Ukraine, Faculty of Food Technology and Quality Control of Agricultural Products, Department of Technologies of Meat, Fish and Marine Products, Heroiv Oborony Str., 15, 03040, Kyiv, Ukraine
Dr Himani Bajaj (Google Scholar)
AVIPS, Shobhit University, Gangoh, India
Dr Njoud Altuwaijri (Google Scholar)
Pharmaceutical Sciences Department, MCPHS University, 179 Longwood Ave, Boston, MA 02115, USA
Dr Laxmidhar Sahoo (Google Scholar)
Centurion University of Technology and Management, Bhubaneswar, Odisha, India
Dr Sukanta Bandyopadhyay (Google Scholar)
Department of Biochemistry, Rama Medical College Hospital & Research Centre, Mandhana, Kanpur-20921, India
Prof Biswajit Dash (Google Scholar)
Amity Institute of Pharmacy, Amity University, Kolkata, West Bengal 700135, India
Dr Apurba Kumar Barman (Google Scholar)
Department of Pharmacy, R. P. Shaha University, Naryanganj, Bangladesh
Dr Thendral Hepsibha B. (Google Scholar)
Department of Biochemistry, Ethiraj College for Women, Chennai – 600 008, Tamil Nadu, India
Prof Ruhul Kuddus (Google Scholar)
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Bangladesh
Dr Gabriel Sunday (Google Scholar)
Federal University Wukari, Taraba State, Nigeria
Dr Pintu Kumar De (Google Scholar)
Department of Pharmaceutical Technology, JIS University, 81, Nilgunj Road, Agarpara, Kolkata-700109, West Bengal, India
Dr Tamer A. Addissouky (Google Scholar)
Department of Biochemistry, Science Faculty, Menoufia University, Egypt
Dr Emmanuel Mfotie Njoya (Google Scholar)
Laboratory of Pharmacology and Toxicology, Department of Biochemistry, Faculty of Science, University of Yaoundé I, 812 Yaoundé, Cameroon
Dr Oky Hermansyah (Google Scholar)
Program Studi D3 Farmasi, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Bengkulu, Bengkulu, Indonesia
Dr Bunu Khatiwara (Google Scholar)
Department of Pharmaceutics, Faculty of Pharmacy, Al Ameen College of Pharmacy, Karnataka 560027, India
Prof Roshan Kumar Dubey (Google Scholar)
Department of Pharmaceutics, Mahatma Gandhi Institute ofPharmacy, Lucknow, Uttar Pradesh, India
Prof Sushil Paliwal (Google Scholar)
Mahatma Gandhi Mission’s Institute of Management and Research, MGM University, Chhatrapati Sambhajinagar, Maharashtra, India
Dr Isna Hikmawati (Google Scholar)
Universitas Muhammadiyah Purwokerto, Indonesia
Dr Mustika Weni (Google Scholar)
Department of Basic Medical Science, Faculty of Medicine, Universitas Swadaya Gunung Jati, Indonesia
Dr Kartikay Prakash (Google Scholar)
Assistant Professor, Lucknow Model College of Pharmacy, Lucknow, Uttar Pradesh, India.
Dr Arushi Sharma (Google Scholar)
Assistant professor, Department of Pharmaceutics, SIP Chandpur 174004, India
Dr Ejiofor InnocentMary IfedibaluChukwu (Google Scholar)
Department of Pharmacognosy and Traditional Medicine, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka-420110, Nigeria
Prof. Dr. Siti Thomas Zulaikhah, SKM., MKes (Google Scholar)
Department of Public Health, Faculty of Medicine, Universitas Islam Sultan Agung, Indonesia.
Prof Opeyemi Joshua Olatunji (Google Scholar)
Faculty of Thai Traditional Medicine,Prince of Songkla University, Hat Yai,90110, Thailand
Prof Alemayehu Toma (Google Scholar)
Department of Pharmacology, Hawassa University, Hawassa, Ethiopia
Dr Shehu Shittu (Google Scholar)
Department of Physiology, University of Ibadan, Ibadan, Nigeria.
Dr Chika Anna Idaguko (Google Scholar)
Department of Anatomy, Faculty of Basic Medical Sciences, Edo State University Uzairue, Nigeria
Dr Anita Lidesna Shinta Amat (Google Scholar)
Department of Biochemistry, Faculty of Medicine and Veterinary Medicine, Universitas Nusa Cendana, Indonesia
Dr Akighir John (Google Scholar)
Department of Biochemistry, Federal University Wukari 200 Katsina-Ala Road, P.M.B 1020 Wukari, Taraba State, Nigeria
Dr Lukman Edwar (Google Scholar)
Department of Ophthalmology, Faculty of Medicine, Universitas Indonesia, Jakarta 10440, Indonesia.
Prof Chandan Mohanty (Google Scholar)
St. Mary’s group of Institutions,Deshmukhi(V), Hyderabad-508284, Telengana, India.
Dr Fikayo Noah Adegboyega (Google Scholar)
Department of Biochemistry, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
Prof Nawsabah Noor (Google Scholar)
Department of Medicine, Popular Medical College, Dhaka-1205, Bangladesh.
Dr Majedul Hoque (Google Scholar)
Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh
Dr apt. Aris Fadillah, M.Farm (Google Scholar)
Faculty of Pharmacy, Islamic University of Kalimantan Muhammad Arsyad Al Banjari, Banjarmasin 70123, South Kalimantan, Indonesia
Dr Chidi Ijeoma Nosiri (Google Scholar)
Department of Biochemistry, Abia State University, Uturu, Nigeria
Dr Benni Iskandar (Google Scholar)
School of Pharmacy, College of Pharmacy, Taipei Medical University, Taipei, Taiwan.
Dr Mita Restinia (Google Scholar)
UIN Jakarta & College of Medicine NCKU, Taiwan
Dr Stephen Olaide Aremu (Google Scholar)
University of Nigeria, Nsukka, Nsukka, Enugu State, Nigeria
Dr Akila Devi D. (Google Scholar)
Department of Pharmaceutics, Research scholar, School of pharmaceutical sciences, Vels Institute of science, Technology and Advanced studies, Pallavaram, Chennai, India.
Dr apt. Nily Su’aida, M.Farm (Google Scholar)
Faculty of Pharmacy, Islamic University of Kalimantan Muhammad Arsyad Al Banjari, Banjarmasin 70123, South Kalimantan, Indonesia
Dr Mahacita Andanalusia (Google Scholar)
Universitas Muhammadiyah Mataram, Kota Mataram, Indonesia
Prof. Dr. Tamader Y. Elghnimi (Google Scholar)
Department of Industrial Pharmacy, Faculty of Pharmacy, Tripoli University, Libya
Dr Syarpin (Google Scholar)
Department of Chemistry Education, Faculty of Education and Teacher Training, Universitas Palangka Raya, Palangka Raya 73112, Kalimantan Tengah, Indonesia.
Dr Chen Van Tran (Google Scholar)
Faculty of Traditional Medicine, University of Medicine and Pharmacy, Ho Chi Minh City, Vietnam
Dr May Thazin Thant (Google Scholar)
Department of Pharmacognosy, University of Pharmacy, Yangon, Myanmar
Dr Anam Razzaq (Google Scholar)
College of Pharmaceutical Sciences, Soochow University, Suzhou 215123, China
Dr Chisom Finian Iroka (Google Scholar)
Department of Botany, Nnamdi Azikiwe University, Awka, Nigeria
Prof Archana S. Patil (Google Scholar)
Department of Pharmaceutics, KLE University’s College of Pharmacy, Nehru Nagar, Belagavi 590010, Karnataka, India
Dr Ahmed Abd El-Moniem Amer (Google Scholar)
Department of Clinical Pharmacy, Damanhur Military Hospital, Behiera, Egypt.
Prof Quratulane Gillani (Google Scholar)
Assistant Professor, The Women University Multan, Pakistan
Dr. Tabarak Malik (Google Scholar)
Department of Biomedical Sciences, Institute of Health, Jimma University, Jimma, Oromia 378, Ethiopia
Dr Ratri Rokhani (Google Scholar)
Pharmacology Laboratory, Faculty of Pharmacy, Universitas Muhammadiyah Purwokerto, Banyumas 53182, Indonesia
Dr Irma Rahayu Latarissa (Google Scholar)
Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia
Dr Shouvik Kumar Nandy (Google Scholar)
Assistant Professor in Pharmacology at Burdwan Institute of Pharmacy, Purba Barddhaman 713103, India
Dr. Mera A. Ababneh (Google Scholar)
Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
Dr Ridho Islamie (Google Scholar)
Departemen Farmasi Klinis dan Komunitas, Fakultas Farmasi, Universitas Surabaya, Surabaya 60293, Indonesia
Dr Mark Danquah (Google Scholar)
Departmentof Pharmaceutical Sciences, Faculty of Applied Science and Technology, Sun-yani Technical University, Sunyani, Ghana
Dr Bachtiar Rivai (Google Scholar)
Pharmaceutical Science and Technology, Chulalongkorn University, Bangkok, 10330, Thailand
Dr. Mrinmoy Chakraborty (Google Scholar)
Department of Pharmaceutical Science, Kumaun University, Nainital 263002, India
Dr. Nesibe Yilmaz (Google Scholar)
Department of Anatomy, Faculty of Medicine, Karabük University, Karabuk, Turkey.
Dr. Sulochana Beeraka (Google Scholar)
Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Chowdavaram, Guntur, Andhra Pradesh, India.
Prof. Dr. Samiullah Khan (Google Scholar)
Center for Eye & Vision Research, 17W Science Park, Hong Kong SAR, China.
Dr apt. Erma Yunita, M.Sc. (Google Scholar)
Akademi Farmasi Indonesia, Yogyakarta, Indonesia
Dr Mia Nisrina Ambar Fatin (Google Scholar)
Fakultas Farmasi, Universitas Bhakti Kencana, Bandung, Indonesia
Dr Sk Mizanul Haque (Google Scholar)
Department of Scientometrics, Library and Information Sciences, University of Calcutta, Kolkata, India
Dr Adhe Septa Ryant Agus (Google Scholar)
Pharmacy Study Program, College of Health Sciences Dirgahayu, Samarinda 75122, Indonesia
Dr Agus Rusdin (Google Scholar)
Department of Pharmacy, Bandung Health Polytechnic, West Java, Indonesia.
Dr Mayang Kusuma Dewi (Google Scholar)
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang 45363, Indonesia
Prof. Dr. Mohd Mujeeb (Google Scholar)
Department of Phytochemisty & Pharmacognosym, Faculty of Pharmacy,Jamia Hamdard, New Delhi 110062, India
Dr Hardika Aditama (Google Scholar)
Department of Pharmaceutics, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia
Dr James H. Zothantluanga (Google Scholar)
Department of Pharmaceutical Sciences, Faculty of Science and Engineering, Dibrugarh University, Dibrugarh 786004, Assam, India
Dr Iksen (Google Scholar)
Departemen Farmasi, Sekolah Tinggi Ilmu Kesehatan Senior Medan, Indonesia.
Prof. Dr. Azhar Mehmood (Google Scholar)
Department of Botany, Hazara University, Mansehra, Pakistan.
Prof. Dr. Zarna R. Dedania (Google Scholar)
Department of Quality Assurance, Bhagwan Mahavir College of Pharmacy, Vesu, Surat, Gujarat, India
Any enquiry related to the journal, please reach us through email below.
Sciences of Pharmacy editorial's email: sciphar@etflin.com (Managing Editor)
Office: Sungai Manonda Street, Duyu, Tatanga,
Palu City 94225, Indonesia.
WhatsApp: +62 82216335184 (Customer Service)
Publisher email: halo@etflin.com (Customer Service)
All papers published in the journal are freely accessible immediately after publication. The papers are freely available to read, shared, and reproduced in any form with proper citation to the original work. Open access is a property of individual works and community standards that will enforce proper attribution and responsible use of the published work.
Publication in this journal requires a processing fee of IDR 1,100,000, which includes taxes and payable after acceptance. There are no additional fees beyond this publication fee. For authors from other countries, PayPal or Wise can be used to automatically convert the amount using real-time exchange rates. A waiver is applicable for authors who fall into these categories.
The journal is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
The authors retain the copyright © of their published works. However, the published work of the
authors can be read, downloaded, shared, and cited with proper reference to the original work.
You may read this article for a better Understanding of Copyright.

Research Article
Open Access
Monday, 16 February 2026
Effect of Tween 80 and Span 80 Surfactants Systems on the Malus domestica Emulsions for Anti-Cutibacterium acnes
Theodorus Rexa Handoyo et al.
Read more
Letter to Editor
Open Access
Thursday, 12 February 2026
Inappropriate Use of Parenteral Analgesics for Mild Pain and Uncomplicated Fever in the Emergency Department: Findings from an Internal Audit
Rissa Maharani Dewi et al.
Read more
Research Article
Open Access
Friday, 6 February 2026
Ethanolic Extract of Curcuma zedoaria Enhances Burn Wound Healing in Male White Rats
Yuliawati Yuliawati et al.
Read more
Research Article
Open Access
Tuesday, 3 February 2026
Health-Related Quality of Life in Patients with COVID-19 in Indonesia: A Cross-Sectional Study
Fajriansyah Fajriansyah et al.
Read more
Research Article
Open Access
Wednesday, 28 January 2026
Efficacy and Safety of Tenofovir in Preventing Perinatal Hepatitis B in Jakarta
Cholid Muzakar et al.
Read more
Review
Open Access
Monday, 19 January 2026
Nanochemistry in Vaccine Delivery: Lipid Nanoparticles, Polymers, and Hybrid Systems
Courage Chandipwisa et al.
Read more
Case Report
Open Access
Tuesday, 6 January 2026
Management of Iodine Contrast Media Related Anaphylactic Shock following Renal Arteriography: A Rare Case Report
Kino Kino et al.
Read more
Research Article
Open Access
Tuesday, 6 January 2026
Animal Models of Acute Exacerbations COPD: Mechanistic Insights and Translational Challenges
Rika Sari Dewi et al.
Read more
Research Article
Open Access
Monday, 5 January 2026
The Relationship Between Medication-Related Burden and Therapy Compliance of Hypertension Patients
Woro Supadmi et al.
Read more
Research Article
Open Access
Wednesday, 31 December 2025
Comparative Glycemic Effectiveness of Long- and Rapid-Acting Insulin in Patients with Type 2 Diabetes Mellitus
Entris Sutrisno et al.
Read more
Research Article
Open Access
Monday, 11 November 2024
Druggability of Pharmaceutical Compounds Using Lipinski Rules with Machine LearningSamukelisiwe Nhlapho et al.
Review
Open Access
Monday, 3 July 2023
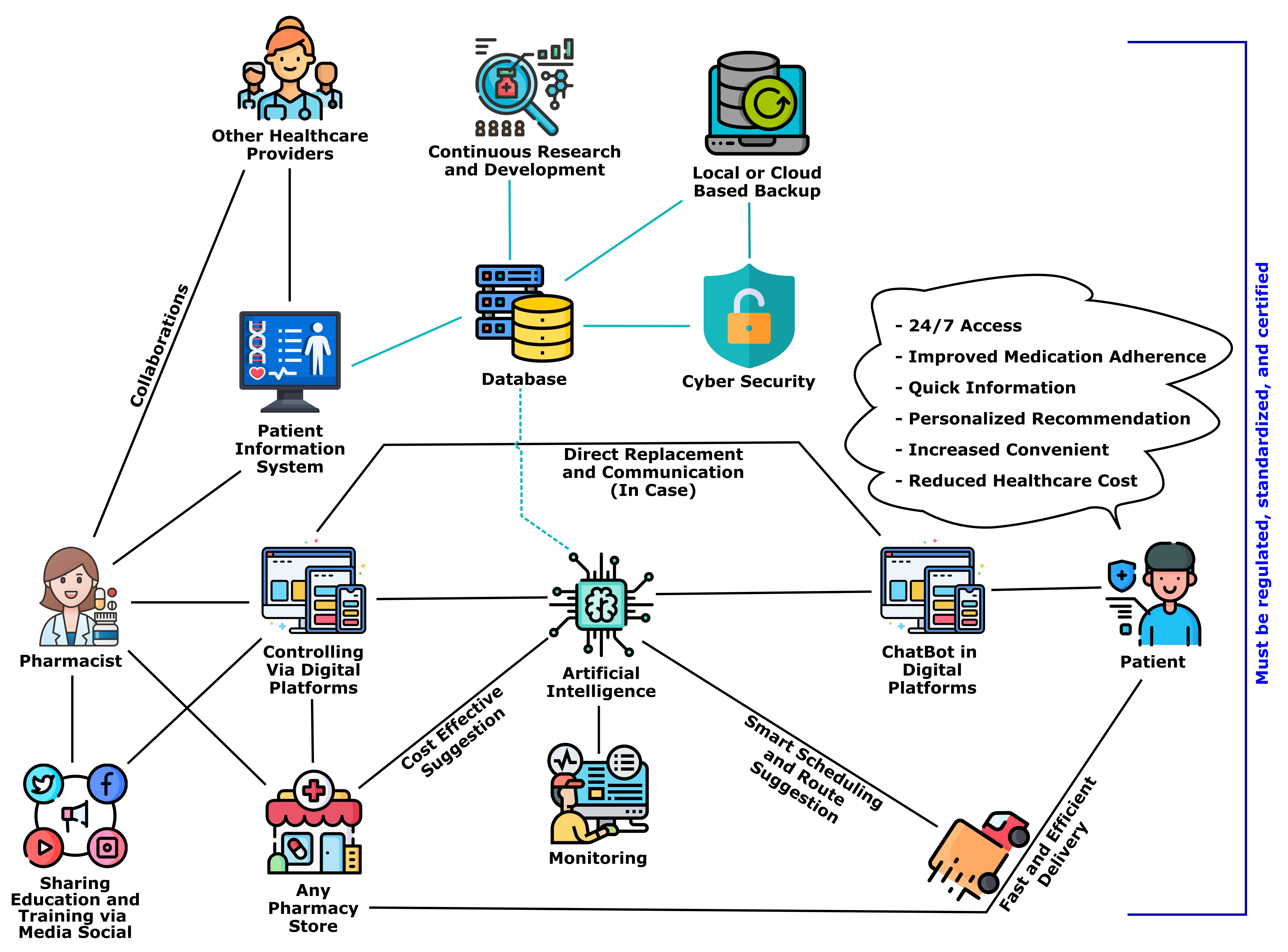
Chatbots in Pharmacy: A Boon or a Bane for Patient Care and Pharmacy Practice?Chusnul Nur Ramadhani et al.
Review
Open Access
Sunday, 25 February 2024
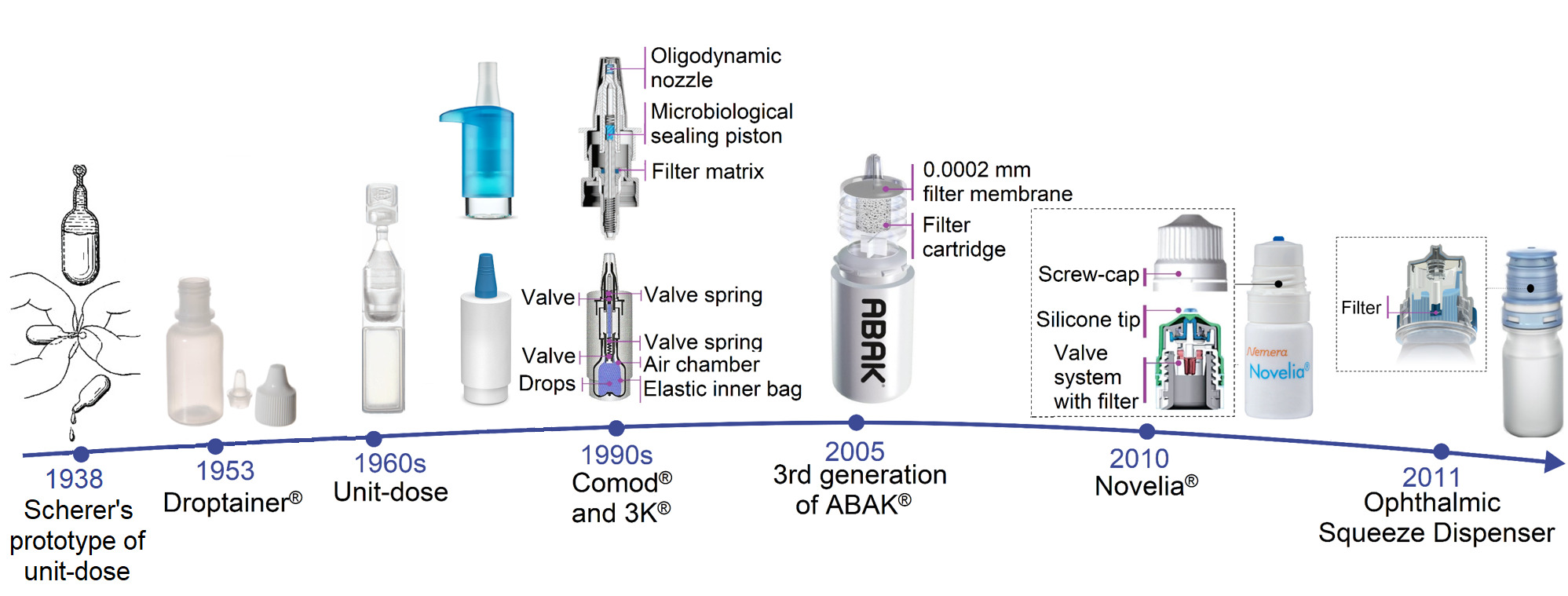
Sterility of Ophthalmic Solutions as a Factor in the Evolution of Primary Packaging for Eye Drops: A Literature ReviewIvan Sergeevich Ivanov et al.

Review
Open Access
Sunday, 26 June 2022
Method and Critical Aspect of Semisolid MixingMeylani Sutoro et al.

Research Article
Open Access
Sunday, 14 January 2024
Prevalence, Causes, and Management Strategies of Fungal Diseases in Northern Regions of BangladeshMd. Khokon Miah Akanda et al.
As a publisher, we create, store, and utilize cookies to enhance the features and services we provide. Several necessary cookies are implemented as part of the website's functionality.
_ga | etflin.com
The purpose of this cookie, set by Google Analytics, is to track the number of site visitors by remembering whether or not you have previously visited our website.
The following cookies are utilized to identify and authenticate users. By utilizing our service and registering an account within our system, you are consenting to the use of the following cookie. We do not share this data to any third party.
username | etflin.com
We employ this cookie to recognize users who have logged into the user system, ensuring that the data you manage is labeled with your username.
email | etflin.com
Email is utilized as our communication tool to send notifications, conduct initial registration verification, and account filtering.
name | etflin.com
We utilize your name to display on the website menu, indicating that you have successfully logged into the system.
token | etflin.com
We use a token to authenticate and identify your login session.
We Revolutionize Sciences, We Publish Sciences, We Are Scientist
ETFLIN