NLP Analysis of Mannan-Based Drug Delivery Trends
by Reza Pratama, Daisy Jane Cabellon-Semense, Lela Sulastri, Mia Arifka, Yayan Rizikiyan ★
Academic editor: Adeleye Ademola Olutayo
Sciences of Pharmacy 4(3): 151-170 (2025); https://doi.org/10.58920/sciphar0403339
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
01 May 2025
10 Jun 2025
15 Jul 2025
23 Jul 2025
Abstract: Mannan, a polysaccharide derived from various sources, has gained attention for its biocompatibility and potential in targeted drug delivery. Since its initial use in 1911 as an ointment base, mannan has been applied in cancer therapy, vaccine development, and as an antimicrobial agent. However, research is still largely dominated by in vitro and preclinical studies, with few clinical trials conducted to date. This study aims to provide an overview of mannan's advancements, its uses in drug delivery, the mechanisms involved, the research gaps, and the underexplored areas with significant potential. This study analyzed 321 peer-reviewed articles selected from Scopus (2000-2024), employing natural language (NLP) and bibliometric mapping to identify key materials, application areas, and research trends. Mannan’s flexible molecular structure allows for copolymerization with polymers such as chitosan, alginate, polyacrylate, and polycaprolactione, enabling improved targeting, mucoadhesion, and controlled drug release. Chitosan emerged as the most frequently used co-polymer, particularly in nanogel formulations for cancer and inflammatory diseases. Keyword impact analysis also revealed growing interest in mannans role in post-COVID-19 cytokine storm mitigation and vaccine enhancement, despite limited representation in clinical pipelines. Optimization of polymer ratios, crosslinker use and formulation strategies, remains essential to improving translational outcomes. Future research should also focus on clinical trials to demonstrate its effectiveness. In conclusion, this study underscores mannans role as promising biomaterial for next-generation drug delivery systems, while identifying gaps in clinical validation, mechanistic insight, methodological consistency. To advance toward commercial and clinical applications, future research should integrate machine learning models for predicting formulation parameters and drug release profiles. This findings offer a roadmap for the design, standardization, and eventual commercialization of mannan-based delivery platforms.
Keywords: MannanNanocarrierTargeted deliveryChitosanImmunotherapyCytokine stormClinical translationMachine learning
Introduction
Polysaccharides have been extensively utilized in various medical applications, particularly as biocompatible materials for drug delivery and therapeutic systems. Common polysaccharides frequently used in this field include cellulose and Chitosan, which serve as structural materials or delivery agents (1, 2). However, these polysaccharides have notable limitations despite their effectiveness in certain applications. For example, cellulose and Chitosan are insoluble in water, requiring additional steps such as chemical modification or organic solvents to improve their solubility (3). These processes add complexity to their preparation, increase the cost, and limit their scalability in industrial applications. Furthermore, the extraction of cellulose and chitosan from natural product sources can be time-consuming and environmentally taxing. This comparison is presented to highlight the need for alternative polysaccharides that overcome these challenges. Identifying polymers with better aqueous solubility, simpler processing, and more sustainable sourcing is essential for advancing drug delivery technologies. As such, mannan has emerged as a promising candidate due to its favorable physicochemical properties and ease of modification (4, 5).
In this context, mannan has emerged as an up-and-coming alternative. Mannan is a naturally abundant, water-soluble polymer that can be extracted easily through hot water or salt solutions, making the extraction process less complex and more environmentally friendly. Mannan is found in many natural sources, including grains, seeds, and Algae, further enhancing its appeal due to its availability and sustainability (6). Unlike cellulose and Chitosan, which require extensive processing, mannan can be utilized naturally for various applications, reducing the need for additional modifications. Structurally, mannan consists primarily of linear or branched chains of mannose units, connected by β-(1→4) or α-(1→6) glycosidic bonds. This configuration provides both flexibility and multiple reactive sites for chemical modification, drug conjugation, or cross-linking(7). These properties allow mannan to form hydrogels, nanoparticles, and other delivery matrices suitable for controlled release. Furthermore, the presence of mannose residues enables receptor-mediated targeting, particularly through mannose receptors on immune cells such as macrophages and dendritic cells making mannan especially valuable in vaccine delivery and immunotherapy.
Mannan's unique properties, including its water solubility and biocompatibility, have made it an increasingly attractive candidate for innovative drug delivery systems such as nanoparticles, microcapsules, and hydrogels. Its digestion by Aspergillus niger β-mannanase in the intestines enables targeted drug delivery to the colon, making it valuable for treating conditions like Crohn's disease and ulcerative colitis (8, 9), as it allows precise delivery with minimal systemic side effects. Mannan's ability to absorb water and swell also makes it suitable for gastric-floating delivery systems, which provide sustained drug release in the stomach, improving efficacy for drugs absorbed in the stomach or upper small intestine (10). Moreover, mannan can be crosslinked with lectin to facilitate the oral delivery of proteins like insulin (11). This offers a potential for non-invasive protein therapies in the digestive tract, where proteins are typically degraded.
Furthermore, mannan has garnered significant attention in immunotherapy due to its ability to engage multiple immune pathways, particularly through its interaction with mannose receptors (MRs) on antigen-presenting cells (APCs) such as dendritic cells, macrophages, and certain T cells. This interaction enhances antigen uptake by APCs, facilitating their processing and presentation via major histocompatibility complex (MHC) class II molecules to CD4+ helper T cells, thereby triggering adaptive immune responses essential for cellular and humoral immunity (12). Mannose receptors are part of the C-type lectin receptor family, which recognize carbohydrate structures on pathogens and promote endocytosis. The ability of mannan to bind these receptors, along with other immune-modulatory pathways like toll-like receptors (TLR4), makes it a potent immunostimulant. By engaging TLR4, mannan stimulates the production of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), enhancing innate and adaptive immunity, which is especially important in antitumor therapies. Mannan's dual capacity to target both MRs and TLRs amplifies the immune response, increasing its immunogenicity in vaccines and therapeutic agents (13).
Additionally, mannan can facilitate the cross-presentation of antigens via MHC class I molecules, leading to the activation of CD8+ cytotoxic T cells, which is particularly valuable in cancer immunotherapy, as these cells can directly target and kill tumor cells. Mannan-conjugated vaccines have shown great promise, particularly in cancer models like melanoma, where they enhance dendritic cell maturation and stimulate robust Cytotoxic T Lymphocyte (CTL) responses, significantly improving therapeutic efficacy (14). Mannan is also involved in immune tolerance, with specific configurations promoting the differentiation of Regulatory T cells (Tregs). These help maintain immune homeostasis and prevent excessive immune responses, highlighting its potential in treating autoimmune diseases. Beyond cancer, mannan has been used in the development of vaccines for infectious diseases such as HIV. In HIV DNA vaccines, mannan enhances antigen delivery to dendritic cells, promoting stronger CD4+ and CD8+ T cell responses (15) .
Despite the promising potential of mannan, there is a noticeable lack of comprehensive reviews that explore its versatility across various therapeutic systems. Review methods also tend to be limited, often analyzing a small selection of literature and offering a narrow perspective. To address these limitations, bibliometric analysis provides a more thorough approach by incorporating a large number of articles for deeper evaluation, particularly with the help of machine learning (16, 17). Traditional bibliometric methods rely heavily on author-supplied or indexed keywords, typically limited to 3-10 terms. Crucially, many important study variables are found in the abstract but are not included in the keywords, making them inaccessible to conventional tools. Machine learning, especially natural language processing (NLP), offers a new solution by enabling the extraction of additional keywords and variables. NLP tools like SciSpaCy, tailored for scientific literature, have gained popularity for their ability to extract terms and phrases (tokens), broadening the dataset and allowing for more detailed exploration of key topics (18, 19). Algorithms such as Latent Dirichlet Allocation (LDA), a probabilistic model for analyzing text, help identify key themes (20, 21). These advancements clarify current trends and research gaps in mannan-based drug and vaccine delivery systems. This study seeks to fill these gaps by offering a broader analysis of mannan's applications in modern drug delivery systems. Specifically, it aims to evaluate the unique advantages of mannan copolymerization, provide insights into its potential scalability, and discuss the challenges that must be addressed to harness its capabilities thoroughly. By examining recent advancements and identifying areas for further research, this study will contribute to a more comprehensive understanding of mannan's role in the future of therapeutic delivery.
Methodology
Search Strategy and Data Collection
A comprehensive literature search was conducted to identify relevant studies using mannans as drug carriers in the Scopus database. The search utilized the keywords "Mannan" in conjunction with "Drug Carrier," "Drug Delivery," or "Delivery System" within the title, abstract, and keywords fields (TITLE-ABS-KEY). The initial search yielded a total of 465 results. To refine the results, 90 reviews, 5 book chapters, 4 notes, 3 conference papers, and 1 survey, editorial, and conference review were excluded, resulting in 360 articles. Next, language restrictions were applied: only articles published in English were included in the analysis, removing 11 documents in Chinese and 1 in German, leaving 348 articles. An additional manual exclusion of 15 articles was performed based on topic relevance and quality. We excluded articles not about mannans (e.g., those focusing solely on the enzyme mannanase), did not involve drug delivery systems, and did not characterize the system. Review articles that passed Scopus' web screening were also excluded. Any material lacking essential metadata, such as abstracts and author/index keywords, was removed. The final dataset includes 321 articles for the analysis. Figure 1 presents a PRISMA-based flowchart illustrating search tactics and outcomes.
Text-Mining, Natural Language Processing, and Data Processing
In bibliometric analysis, the results are based on the keywords provided by the authors or those indexed. However, these keywords often fail to cover a wide range ofessential study variables, such as dosage forms, drugs, copolymers, crosslinkers, target diseases, and other specific parameters necessary for trend analysis. To identify more precise keywords with these characteristics, text mining was performed in two ways: 1) manually gathering related keywords/categories by reading titles and abstracts, and 2) using the Named Entity Recognition (NER) algorithm from the SciSpaCy package (en_core_sci_sm model) applied to the final dataset. The NER process was carried out using a custom Python module, where filtering was applied to remove non-noun words like 'efficient' or 'increase,' and exclude generic search or dataset-related terms. The extracted keywords were categorized into five thematic groups: copolymers, active pharmaceutical ingredients (APIs), particle shapes, diagnostic methods, and therapy types.
These keywords were then analyzed using Latent Dirichlet Allocation (LDA) to identify dominant research topics. For reproducibility, the LDA model was built using the Gensim library (v4.3.0) with the following parameters: number of topics = 20, number of keywords per topic =5, coherence score = 0.317 (evaluated using the C_V method), passes =10, iterations =1000, and random_state =42. Preprocessing included tokenization, lemmatization, stopword removal, lowercasing, and removal of punctuation and numbers. A TF-IDF vectorizer was used with min_df = 5 and max_df = 0.7 to filter out rare and overly common terms. Additionally, keywords directly related to inclusion criteria from the literature search were excluded to refine the topic output. This approach ensured that the topic modeling process was both systematic and reproducible.
In this study, certain tasks that existing tools could not manage were handled using custom Python modules. These processes included converting a scanned and filtered database into a CSV format compatible with Biblioshiny, calculating the frequency of NER-extracted keywords in the dataset, creating a keyword timeframe dataset, and computing the keyword impact described in Equation 1. The keyword impact, defined as the ratio of total citations to keyword frequency, was used to identify terms that, while not frequently appearing, were associated with highly cited studies thereby highlighting influential or emerging research themes that may not be captured by frequency alone.
The bar plot for topics generated by LDA was visualized with Python's pyLDAvis and matplotlib packages. Meanwhile, the timeframe, keyword impact, and radial network were visualized using the ggplot and Network3D packages in RStudio (Version 4.2.1, RStudio Inc, Boston, USA).
Research Questions
The research questions addressed in this study include: What types of mannan are utilized in drug delivery systems, and how do their unique properties enhance therapeutic outcomes? What other polymers are commonly used in combination with mannan, and how do these combinations improve the efficiency and targeting of the delivery system? What types of delivery systems can be developed from mannan, and why is it crucial to explore innovative methods for drug administration? What diseases can be targeted using mannan-based systems, particularly concerning its immunogenic properties and potential for treating complex conditions? Lastly, what are the recent advancements or revolutions in mannan-based delivery systems, and how do they contribute to overcoming current challenges in drug delivery technologies? Answering these questions is essential for understanding how mannan can be optimized in biomedical applications.
These questions form the foundation for a comprehensive evaluation of mannan’s role in modern therapeutics. By identifying key materials, mechanisms, and clinical targets, the study aims to highlight both the opportunities and limitations of mannan-based approaches. This focused analysis also opens the door for future research into hybrid systems and emerging biomedical technologies.
Mannans Definition, Types, and Manufacturing
Structure and Source
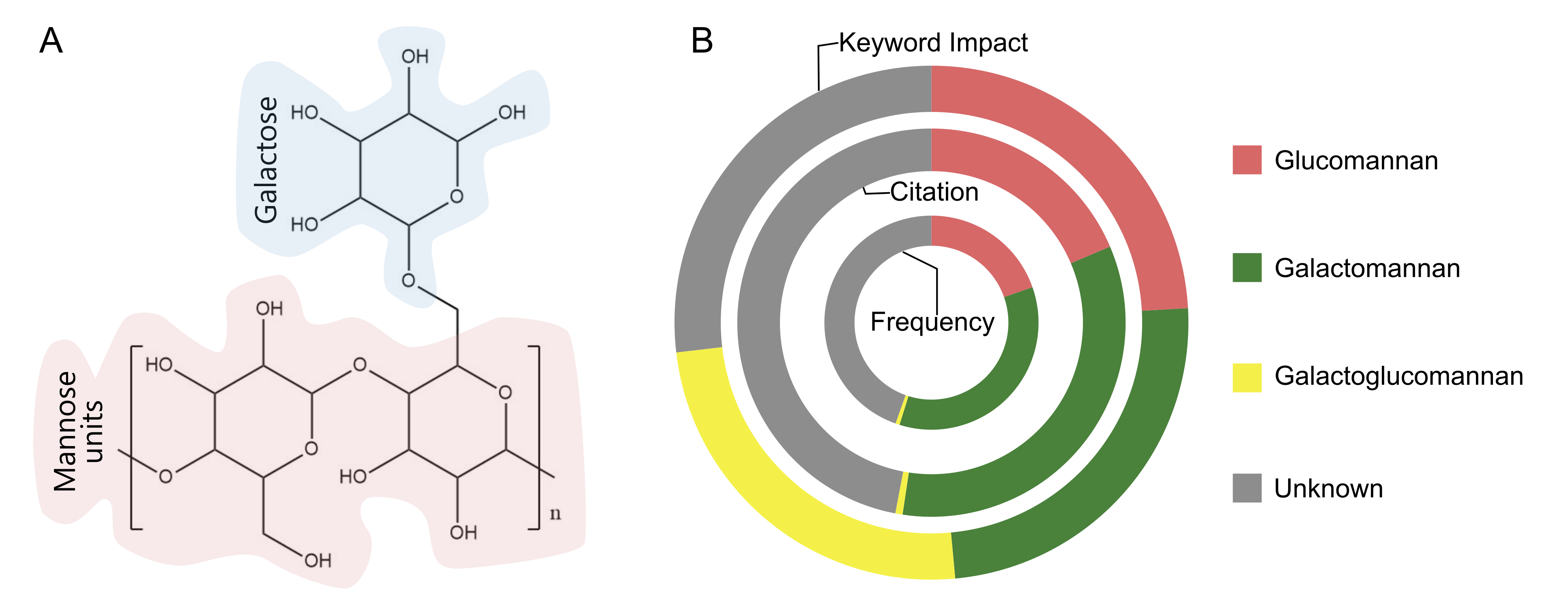
Mannans are polysaccharides composed primarily of mannose residues, a six-carbon sugar (C₆H₁₂O₆), typically linked by β-1,4-glycosidic bonds to forming long chains, with occasional β-1,3 or β-1,6 linkages (22). These structure maybe linear or branched, with side chains that can include mannose or other sugars such as glucose, galactose, or arabinose (see Figure 2A).
Mannans from yeast cell walls have gained increasing attention for their health and cosmetic applications due to their antioxidant, immunomodulatory, cholesterol lowering, and gut health benefits (23). In pharmaceuticals, mannans are widely used in drug delivery systems, where different types offer unique properties. Glucomannans, consisting of β-1,4-linked mannose and glucose reisudes, are the most common, accounting for approximately 42% of mannan-based drug delivery applications due to their biocompatibility and gel forming ability (24). Gallactomannans, with β-1,4-linked backbone and α-1,6-linked galactose side chains, represent about 30% of applications and are valued for their solubility and use in controlled-released formulations. Galactoglucomannans, comprising mannose, glucose, and galactose residues, contribute to around 15% of drug delivery applications, particularly in targeted delivery systems. Yeast mannans, though less frequent (about 10%), are gaining are interest for their immunological targeting capabilities, especially in vaccine and anti-inflammatory formulations. Linear mannans are the least used (under 3%) due to their limited solubility and functional versatility. Mannans are derived from diverse biological sources such as algae (kelp, dulse, nori) fungi, yeast and bacteria including cyanobacteria and Rhizobium (25). To further optimize their performance, mannans are often chemically modified via cationization, oxidation, carboxymethylation, or esterification, to enhance their physicochemical properties for specific drug delivery applications, as discussed in the following section.
Type of Mannans
Based on Sugar Composition (Without Modification)
Glucomannans
Recent studies show that glucomannan has various advantages in pharmaceutical and biomedical applications. The glucomannan basic structure can be seen in Figure 3. Its biocompatibility and biodegradability make glucomannan an attractive material for drug formulations. It can be degraded by β-mannosidase enzymes found in the lungs, as seen in research on glucomannan microparticles used as carriers for anti-tuberculosis drugs (26). These microparticles exhibit high drug association efficiency, ranging from 66% to 91%, with an aerodynamic diameter of around 3 µm, allowing deep lung penetration. Additionally, they can control drug release, with rifabutin being released more slowly than isoniazid, though both are fully released within 24 h (27, 28).
Another study showed that macrophages can absorb glucomannan microparticles, which are crucial for tuberculosis treatment. These microparticles did not exhibit significant cellular toxicity nor trigger systemic or lung inflammatory responses in rats, indicating that glucomannan is safe for pulmonary applications (29). Glucomannan microparticles produced through spray-drying techniques also demonstrated high drug association efficiency. They showed no toxicity in Calu-3 and A549 cells, making glucomannan suitable for creating inhalable microparticles for pulmonary tuberculosis therapy (27). Modified glucomannan (cBSP) also showed a high affinity for macrophages and enhanced gene transfection efficiency, making it a promising candidate for anti-inflammatory gene delivery therapy (29).
In the context of curcumin delivery, glucomannan exhibits good stability and provides adequate protection for curcumin. Research shows that glucomannan can enhance the bioavailability of curcumin by demonstrating good thermal stability and stability in vitro and in vivo. This enables targeted delivery to the colon and improves the effectiveness of curcumin as a biologically active compound (30). Glucomannan also shows great potential for drug delivery applications to the colon. Hydrogel glucomannan, linked with olsalazine, can degrade quickly, matching the retention time in the colon and ensuring faster and more efficient drug release in the colonic environment (31). The degradation of this hydrogel controls drug release, such as 5-fluorouracil, showing improved effectiveness in targeted colon drug delivery (32). Additionally, glucomannan-based calcium delivery systems demonstrate a longer floating time and slower, controlled calcium release, enhancing calcium bioavailability compared to commercial calcium tablets (33).
Galactomannans
Due to its unique physicochemical properties, Galactomannan (GAL) is widely used as a drug delivery system. For example, a study preparing and characterizing fluoxetine microparticles with galactomannan found that these microparticles had an encapsulation efficiency (EE%) of 98%. They were able to release about 60% of the drug within 200 min. The study also indicated that fluoxetine encapsulated in galactomannan microparticles has potential as an effective antimicrobial agent against methicillin-resistant Staphylococcus aureus strains, showing significant results in MIC tests and biofilm activity (34).
Galactomannan is also used for environmentally friendly modification of superparamagnetic iron oxide nanoparticles (SPIONs), with the antineoplastic agent Methotrexate (MTX) covalently bonded to galactomannan. These nanoparticles exhibit drug release kinetics sensitive to reduction and selectively accumulate MTX in tumor cells. The study demonstrated superior tumor reduction and significant survival benefits in mice with Ehrlich ascites carcinoma (EAC) tumors. The nanoparticles also provided excellent contrast in magnetic resonance imaging (MRI) confirmed in syngraft and xenograft mouse models, highlighting their significant potential for clinical use (35).
Additionally, galactomannan extracted from the endosperm of Trigonella persica shows a high M/G ratio of about 5:1, making it suitable for drug delivery systems due to its viscous nature and gel-forming ability. Changes in the physicochemical properties of galactomannan during germination suggest that galactomannan from ungerminated seeds may be more suitable for drug delivery applications (36). Galactomannan is also extracted from the endosperm of Delonix regia and used to develop nanoparticles with potential for ocular drug delivery. These nanoparticles are stable in simulated lacrimal fluid and lysozyme and can release the drug continuously. The research indicates that these nanoparticles are non-toxic to retinal and corneal epithelial cells. Retinal cells can take them up, making them a safe and promising tool for ocular drug delivery (37).
Aerogels obtained from enzymatic oxidation of galactomannan from Fenugreek, Sesbania, and Guar show significantly increased viscosity, producing elastic and stable hydrogels. These aerogels can absorb active principles from aqueous solutions and release them in suitable media, showing potential as versatile and biocompatible biomaterial delivery systems for biomedical and industrial applications (38). Galactomannan from Senna tora seeds also shows potential as an excipient in slow-release drug delivery systems. This galactomannan has a basic structure consisting of a β-d-mannopyranosyl main chain linked via (1→4), with galactopyranosyl units linked via α-(1→6). Rheological studies show that Senna tora gum solutions exhibit pseudoplastic flow, confirming its suitability as an excipient in developing slow-release drug delivery systems (39).
Cationized Mannans
The cationization of mannan polymers is a strategic modification employed to enhance the efficiency and specificity of drug and gene delivery systems across various biomedical applications. This includes specific targeting of macrophages, hepatic cells, and Mesenchymal stem cells (MSCs), while promoting improved cellular uptake and therapeutic efficacy. The cationization process involves introducing positively charged group such as spermine, polyethyleneimine (PEI), or quaternary ammonium to the mannan backbone, thereby increasing its electrostatic interaction with negatively charged nucleic acids and cell membranes. For instance, in one study, cationized mannans modified extracellular vesicles were used deliver docorubicin, where the saturation of the mononuclear phagocyte system (MPS) significantly enhanced drug accumulation in tumor tissues and improve antitumor efficacy (40) Another study employed spermine-modified mannan as a non-viral vector for gene delivery, where it facilitated the transfection of plasmid DNA encoding interleukin-12 (pIL-12) into murine macrophages, resulting in enhanced gene expression (41). In hepatic targeted gene therapy, cationized mannan has been used to deliver siRNA targeting hepatitis B virus (HBV), achieving high transfection efficiency with minimal cytotoxicity (42). From the stem cell applications, cationized polysaccharides including mannan have shown promise in delivering GFP-tagged reporter plasmids to MSCs, where transfection efficiency varied depending on cationic group used and its degree of substitution (43). Additionally, cationized bioactive glucomannan derived from Bletilla striata was utilized to deliver VEGF plasmid for gfene-assisted cell therapy, demonstrating high affinity for macrophages and promoting targeted delivery in inflammatory conditions (44). These examples highlight the versatile application of cationized mannans in delivering both drugs and genetic materials, underscoring their potential in the development of advanced, non-viral, and cell targeted delivery systems.
Oxidized Mannans
The primary goal of oxidizing mannans, such as those derived from Konjac Glucomannan (KG) and other sources, is to introduce reactive groups that facilitate further chemical modifications and crosslinking. This process transforms the naturally occurring polysaccharides into versatile materials capable of forming hydrogels, aerogels, and other composite structures with improved mechanical properties, stability, and functionality. For instance, TEMPO-oxidized galactomannans have been utilized to create aerogels that act as biocompatible delivery systems for antibiotics, enzymes, and other active principles. These aerogels exhibit significant increases in viscosity upon oxidation, forming stable hydrogels that can release therapeutic agents in a controlled manner (45). Similarly, the oxidation of KG has been employed to create light-responsive and pH-sensitive delivery systems. The oxidized polymers, crosslinked with ferric ions, form strong gels that degrade under light exposure, allowing for the controlled release of encapsulated substances (46) and enhancing their potential as an antianemic agent In Vivo (47). Oxidized galactomannan polysaccharides facilitate grafting with aminated poly(N-isopropyl acrylamide) (PNIPAm-NH2), improving their self-organizing ability into nanoparticle copolymers. These copolymers, characterized by their biocompatibility, cell viability, and controlled drug release capabilities, utilize free aldehyde groups post-oxidation for potential pro-drug formations, exemplifying their versatility in therapeutic applications (48).
Another significant aim of mannan oxidation is to enhance the targeting and efficacy of drug delivery to cancer cells. Mannose receptors, which are overexpressed in certain cancer cells, can be targeted by conjugating drugs to oxidized mannans. For example, polymannose-doxorubicin conjugates have shown superior targeting efficacy and cytotoxic potential against cancer cells compared to non-targeted drug formulations (49). This strategy leverages the oxidized mannose groups to specifically bind to mannose receptors on cancer cells, improving drug uptake and retention. Oxidized konjac glucomannan-based microspheres encapsulating α-lactalbumin peptosomes and miR-31 inhibitors demonstrate targeted delivery capabilities in colorectal cancer therapy, leveraging mucoadhesive and mucus-penetrating properties for enhanced local bioavailability and therapeutic efficacy (50).
Oxidized mannans also play a pivotal role in developing non-viral gene delivery systems for cancer immunotherapy. Researchers have enhanced these vaccines' immune response and efficacy by conjugating DNA vaccines to oxidized mannans. For instance, mannan-modified adenovirus vectors have demonstrated improved targeting and reduced off-target effects, making them promising candidates for cancer vaccine delivery (51-54). These conjugates can induce robust T-cell responses, leading to tumor protection and increased survival in animal models.
In addition to drug and gene delivery, oxidized mannans are employed in preparing composite hydrogels for tissue engineering and controlled release applications. Composite hydrogels formed by oxidized konjac glucomannan and carboxymethyl Chitosan show potential as advanced wound dressings with antibacterial properties and can accelerate wound healing by modulating inflammatory responses and promoting tissue regeneration (55). Furthermore, oxidized KG microneedle patches loaded with CuGA-MOF exhibit multifunctional properties for chronic wound healing, combining antibacterial and antioxidant effects while promoting macrophage polarization and angiogenesis (56).
However, despite these promising applications, oxidized mannans may also present off-target effect limitations that must be carefully considered. The introduction of reactive aldehyde groups increases the potential for nonspecific interactions with endogenous biomolecules, such as amine containing proteins and cell surface receptors, which may alter biodistribution or trigger unintended immune responses. At high degrees of oxidation, these mannans may lose their natural biocompatibility and result in cytotoxicity, inflammation, or premature degradation in vivo. Such off-target effects can compromise the therapeutic efficacy and safety. Therefore, controlling the oxidation level and thoroughly evaluating the physicochemical and biological properties of oxidized mannans including cytotoxicity, hemocompatibility, and immune reactivity, is critical for optimizing their design for targeted applications.
Carboxymethylated Mannans
The carboxymethylation of mannoses, such as galactomannan and glucomannan, is carried out to enhance the solubility of these materials in water, which is crucial for their application in drug delivery systems. For example, carboxymethylated galactomannan derived from Fenugreek (57) and Cassia obtusifolia (58) has been shown to improve the solubility and chemical stability of encapsulated active compounds. This enhancement significantly boosts the bioavailability of poorly soluble drugs such as erlotinib and diclofenac sodium, thereby increasing therapeutic efficacy. Moreover, the introduction of carboxymethyl groups enables stronger and mor stable cross-linking with other polymers, contributing to sustained and controlled drug release profiles. These cross-linkages have been successfully applied in nanogels and hydrogels to encapsulate compounds such as Caffeic acid, Eugenol (59), and Curcumin (60), with resulting delivery systems demonstrating uniform particle size, high encapsulation efficiency, and long-term release capability.
In addition to drug capacity, carboxymethylated mannans exhibit improved mechanical and physicochemical properties, including enhanced stiffness, elasticity, and water retention, due to electrostatic interactions and hydrogen bonding. For instance, pH-sensitive hydrogels based on carboxymethylated konjac glucomannan (CMKGM) can be tuned to release their payload at specific gastrointestinal sites such as the intestine or colon (61). Furthermore, nanogels formulated from CMKGM and chitosan show high biocompatibility, including serum stability and hemocompatibility, which are critical for systemic administration (62).
Importantly, the physiological stability of carboxymethylated mannans is a crucial factor in ensuring their performance in biological environments. These derivate maintain structural integrity under physiological pH and iconic strength, protecting encapsulated drugs from premature degradation. Their resistance to enzymatic hydrolysis, particularly in the upper gastrointestinal tract, allows for site-specific release, especially in the colon. For example, CMKGM-based nano-delivery system for naringin or ovalbumin function effectively in neutral aqueous solutions without requiring chemical crosslinkers, demonstrating stability and functionality in stimulated biological fluids (63, 64). Similarly, CMKGM-Chitosan (CMKGM-CS) nanogels developed for probiotic delivery to the intestine support the growth of Lactobacillus reuteri and show resilience against acidic and enzymatic conditions, with in vivo studies confirming effective intestinal release (65). The degree of substitution of carboxymethyl groups is a key determinant of physiological stability, as it influences coacervation, muchoadhesion, and resistance to gastrointestinal degradation (66, 67).
In ocular drug delivery, bioadhesive nanoparticles formulated with carboxymethylated Leucaena leucocephala galactomannan (LLG) sustained the release dorzolamide hydrochloride while enhancing corneal permeation (68). Biodegradable polymer nanospheres of CKGM and hydroxypropyl trimethyl ammonium chloride chitosan (HACC) has shown promising results as vaccine delivery systems, offering high encapsulation efficiency and controlled antigen release (69). Additionally, composite beads combining carboxymethylated Fenugreek galactomannan with gellan gum and calcium silicate effectively delivered glimepiride, demonstrating controlled release and significant hypoglycemic activity in diabetic rats (70). Overall the chemical stability, bioadhesiveness, and physiological compatibility of carboxymethylated mannans make them highly attractive for diverse therapeutic applications, especially where sustained and targeted delivery is critical.
Esterified Mannans
O-palmitoylmannan
Using O-palmitoylmannan (OPM) in polymer-based drug delivery systems represents a significant advancement in targeted therapy, particularly for disease involving macrophages and antigen-presenting cells, such as systemic infections and certain cancers. In antifungan antileishmanial treatments, studies have shown that modifying liposoms and emulsomes with OPM enhances drug accumulation within infected macrophages, thereby improving therapeutic efficacy. For instance, OPM-coated liposomes and emulsomes loaded with Amphotericin B (AmB) demonstrated elevated drug concentrations in macropaghes-rich tissues like the lungs, liver, and spleen (71), which is critical in managing diseases such as pulmonary aspergillosis and visceral leishmaniasis (VL). Animal studies confirmed these system resulted in higher drug retention and reduced infection burden compared to unmodified formulations (72, 73).
In the context of vaccine delivery, OPM-coated niosomes have been formulated for oral and topical administration, showing excellent stability in gastrointestinal fluids and high affinity for mucosal immune sites such as Peyer’s patches and Langerhans cells. These system elicited strong systemic (IgG) and mucodal (IgA) immune responses, offering promise as a cost-effective and thermally stable alternative to traditional cold-chain-dependent vaccines (74, 75). Additionally, OPM coating improves the solubility, stability, and tissue targeting ability of encapsulated drugs, ensuring precise delivery to macrophage-rich environments, while reducing systemic toxicity often associated with conventional antigungan agent (76) (77, 78). Comparative biodistribution studies further support OPM efficacy, showing significantly higher drug accumulation in the liver, spleen and lungs compare to noncoated system (78-80).
Compared to polyethylene glycol (PEGylation), a widely used surface modification strategy, OPM offers a distinct advantage in cell specific targeting. While PEGylation enhances circulation time by preventing opsonization and renal clearance, it lack active targeting capability and may reduce cellular uptake, particularly by immune cells (81). In contrast, OPM leverages mannose receptor-mediated endocytosis, allowung for selective delivery to macrophages and dendritic cells, which is especially valuable in infections and cancer immunotherapy (82). Moreover, repeated administration of PEGylated systems can lead to the accelerated blood clearance (ABC) phenomenon, and anti PEG antibodies have been reported, potentially compromising therapeutic effectiveness. OPM-based systems, being biologically derived and immunomodulatory, may circumvent these drawbacks by combining targeting specificity with innate immune compatibility (83). Thus, OPM severs not only as a stabilizing and solubilizing agent but also as biologically active targeting ligand, offering both pharmacokinetic and pharmacoynamic benefits over traditional PEG-based modifications.
Acrylated Mannans
Acrylated mannans, such as those derived from guar gum and konjac glucomannan, are increasingly favored as drug-delivery polymers due to their unique properties showcased across several studies. For instance, in Reis et al. report (84), the chemically modified guar gum hydrogels demonstrate pH-responsive behavior and enhanced biocompatibility, essential for safe biomedical applications. These hydrogels exhibit controlled drug release characteristics, with a mechanism of drug 5-aminosalicylic acid (5-ASA) studied by different mathematical kinetics models. 5-ASA is a therapeutic drug that cures mild to moderate inflammatory bowel disease, and its pH-sensitive swelling behavior enables effective drug delivery in gastrointestinal conditions (85). Furthermore, based on the research by Kai A. et al. (2020) illustrates how konjac glucomannan-based molecularly imprinted polymers (MIPs) offer selective adsorption and release capabilities, particularly beneficial for targeted anticancer therapies like 5-fluorouracil (86). The development of nanocomposite hydrogels incorporating methacrylate-modified O-acetyl-galactoglucomannan and thiolated cellulose nanocrystals demonstrates their potential as mechanically robust and bioactive matrices suitable for sustained therapeutic ion release (87). Additionally, the formation of nanogels from amphiphilic mannan by the Michael addition of hydrophobic 1-hexadecane-thiol to vinyl methacrylate mannan originates in aqueous medium the formation of a nanogel, stabilized by hydrophobic interactions among alkyl chains, highlight their stability and ability to encapsulate therapeutic molecules like curcumin, thus presenting promising avenues for future therapeutic delivery systems (88).
Acetylated Mannans
Acetylated mannans, derived from natural sources like Aloe vera, are increasingly recognized for their potential as effective drug-delivery polymers in biomedical applications. Research by Rodrigues et al. (2021) discusses the integration of acemannan (ACE), the main polysaccharide from Aloe Vera, into blended films with Chitosan and Alginate (89). These films exhibit stable polyelectrolyte structures due to strong intermolecular interactions, offering enhanced dimensional stability, flexibility, and controlled ACE release profiles suitable for tissue engineering applications. The films also demonstrate biocompatibility with L929 cells, suggesting their suitability as bioactive platforms in tissue engineering. Meanwhile, Li L. et al. (2022) explore the anti-inflammatory properties of polymeric acemannan (ABPA1) isolated from Aloe vera barbadensis extract. ABPA1 significantly inhibits proinflammatory cytokine release and alleviates cytokine storms in various models of inflammatory diseases. The mechanism involves the modulation of macrophage polarization and enhancement of mitochondrial metabolism via the PI3K/Akt/GSK-3β signaling pathway (90).
Sustainable Manufacturing of Mannans
The development of sustainable manufacturing methods for mannans is essential to support their widespread use in biomedical and pharmaceutical applications while aligning with global environment objectives. This methods aim to minimize the use of hazardous chemicals and energy, enhanced resource effeiciency and ensure scalability. The most widely used approaches include hot water extraction, alkaline extraction, enzymatic hydrolysis, microbial fermentation, integrated biorefinery systems. The following subsections provide an overview of these methods, including their extraction efficiency, environmental impact, and estimated production cost where data available.
Hot Water Extraction
Hot water extraction is considered one of the most eco-friendly and cost effective techniques for isolating mannans from plant-based materials. It involves the use of water at elevated temperatures between 80°C to 100°C to disrupt plant cell walls and solubilize mannans. This method is particularly effective for sources such Ceratonia siliqua (locust bean gum) and Amorphophallus konjac (konjac glucomannan).
A study by Ahn et al. (2017) demonstrated the hot water extraction effectively preserved the functional characteristics of konjac glucomannan, such as its high viscosity and water-holding capacity, which are critical for pharmaceutical formulations. The process avoids the use of harmful solvent, leading to minimal environment burden. Yields from hot water extraction typically range from 30% to 60% depending on the raw material and process parameters, process enhancements using ultrasonical or high pressure homogenization have been shown to increase yield by up to 20% without comprimissing sustainability (91).
Salt or Alkaline Extraction
Another sustainable approach involves using mild salt or alkaline solutions to extract mannans. Alkaline extraction works by swelling the plant cell walls, allowing mannans to diffuse into the solution. Sodium hydroxide is often used in this process at low concentrations to minimize environmental impact. Compared to traditional chemical methods, this approach uses less aggressive chemicals, reducing energy and water usage.
For example, Khan et al. (2018) reported the successful extraction of galactomannans from Trigonella foenum-graecum (fenugreek) seeds using a combination of alkaline treatment and ethanol precipitation, yielding up to 65% of high-purity mannans. This method can be further optimized by integrating membrane filtration, thereby reducing solvent use and enhancing downstream purification (92). This approach can also be combined with filtration techniques like membrane filtration, reducing the need for solvents and other chemicals.
Enzymatic Extraction
Enzymatic extraction is considered one of the most specific and environmentally friendly methods for mannan production. Enzymes such as β-mannanase selectively hydrolyze plant materials, breaking down the complex cell wall structures and releasing mannans into solution. This method has a significant advantage in avoiding using toxic chemicals entirely, making it highly sustainable. Moreover, using enzymes allows for a high degree of control over the extraction process, ensuring the integrity and bioactivity of the extracted mannans.
Research by Song et al. (2020) explored the use of β-mannanase in the enzymatic extraction of mannans from palm kernel cake, a waste by-product of the palm oil industry, and demonsrated the enzymatic extraction of mannans from palm kernel cake using β-mannanase, achieving yields of up to 85%. The study showed that enzymatic extraction not only improved yield but also offered a sustainable way to valorize agricultural waste, aligning with the principles of a circular economy (93). The specificity of enzymatic hydrolysis also ensures minimal degradation of the polysaccharides, preserving their molecular weight and functional properties.
Microbial Fermentation
Microbial fermentation offers a bio-based route to producing mannans, particularly through bacteria, fungi, or yeast. This method has gained attention for its potential to use renewable feedstocks, such as agricultural residues, which are fermented by microorganisms to produce mannans. This highly sustainable approach converts waste materials into valuable biopolymers with minimal environmental impact. Furthermore, fermentation processes generally require lower energy input than chemical extraction methods.
A key study by Baek et al. (2019) investigated the production of mannan by yeast species like Kluyveromyces lactis using agricultural residues. Yields typically range from 10 to 30 g/L in batch cultures, depending on microbial strain and fermentation conditions. The fermentation process produced significant yields of mannans with potential applications in the pharmaceutical and food industries. (22). The study emphasized that microbial fermentation could be scaled up for industrial production while reducing reliance on traditional plant-based extraction methods This bio-based approach aligns well with the concept of green chemistry and sustainable biomanufacturing.
Biorefinery Approach
The biorefinery approach integrates the extraction of mannans with the recovery of other valuable components, such as lignin, cellulose, and proteins from biomass. This holistic process aims to maximize the utilization of raw materials and minimize waste, making it a highly sustainable method for manufacturing mannans. In a biorefinery, the entire biomass is fractionated into its constituent parts, and mannans are isolated as part of a broader suite of products, enhancing the overall economic viability of the process.
Bohutskyi et al. (2018) highlighted the potential of biorefineries in their work on extracting mannans from Algae within an integrated system that also produced biofuels and other bioproducts (94). The study showed that Algae-based biorefineries could provide a renewable and scalable source of mannans, with the added benefit of contributing to renewable energy production. By utilizing the full potential of biomass, the biorefinery approach exemplifies a circular and sustainable method for producing mannans. Yields of mannans in biorefinery setups can reach up to 90%, depending on the fractionation efficiency.
Mannan Copolymerization
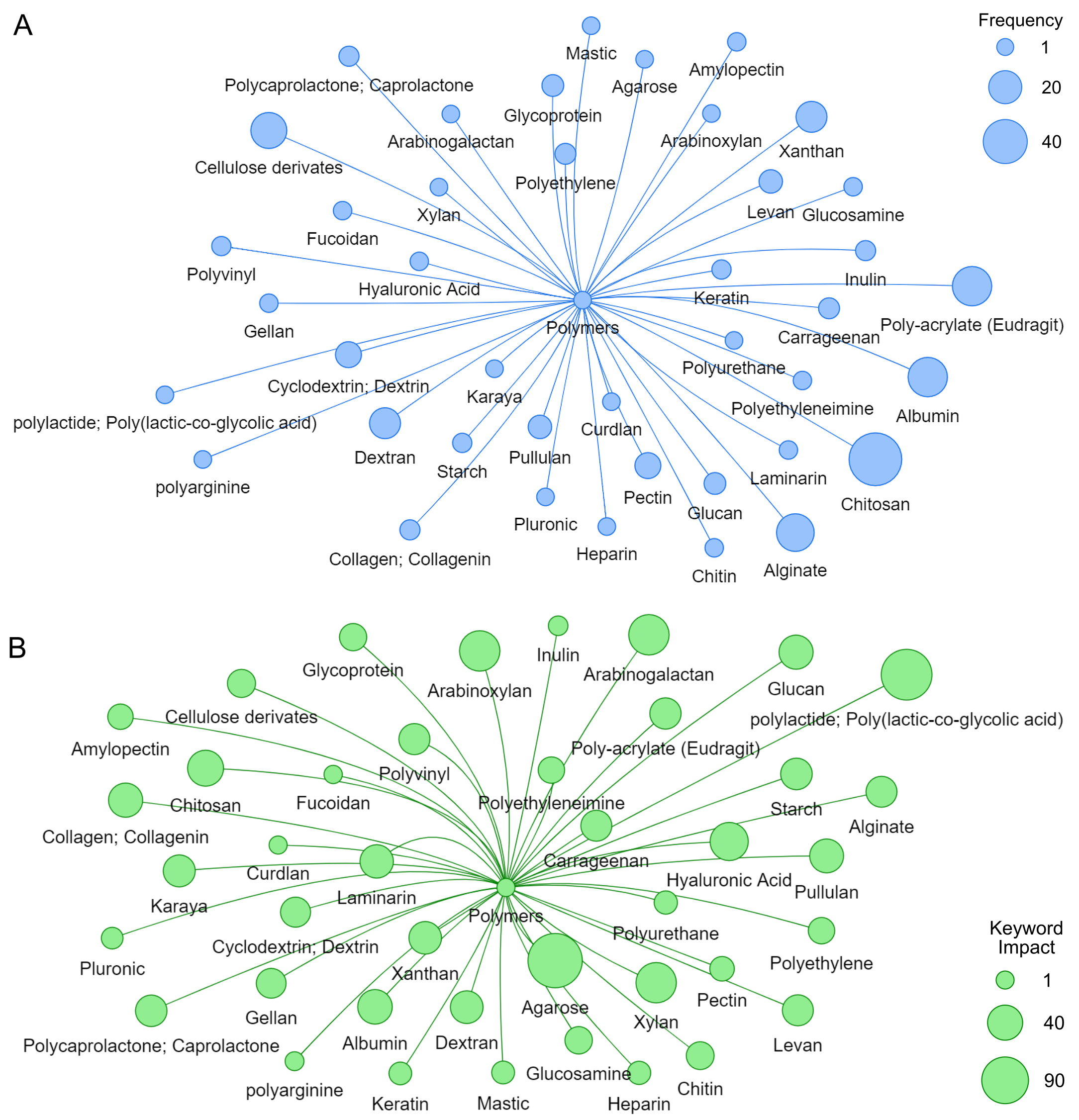
Based on data extracted using NER, 41 major classes ofpolymers were identified for copolymerization with mannan. While some have been used infrequently, they have garnered significant attention within the academic community, as evidenced by their extensive referencing. Common natural polymers include Chitosan, Alginate, cellulose, dextran, and xanthan gum, while synthetic polymers such as polyacrylates, polyethylene, and polycaprolactone are also frequently utilized. Here is an overview of several of these commonly employed polymers.
Based on Usage Frequency
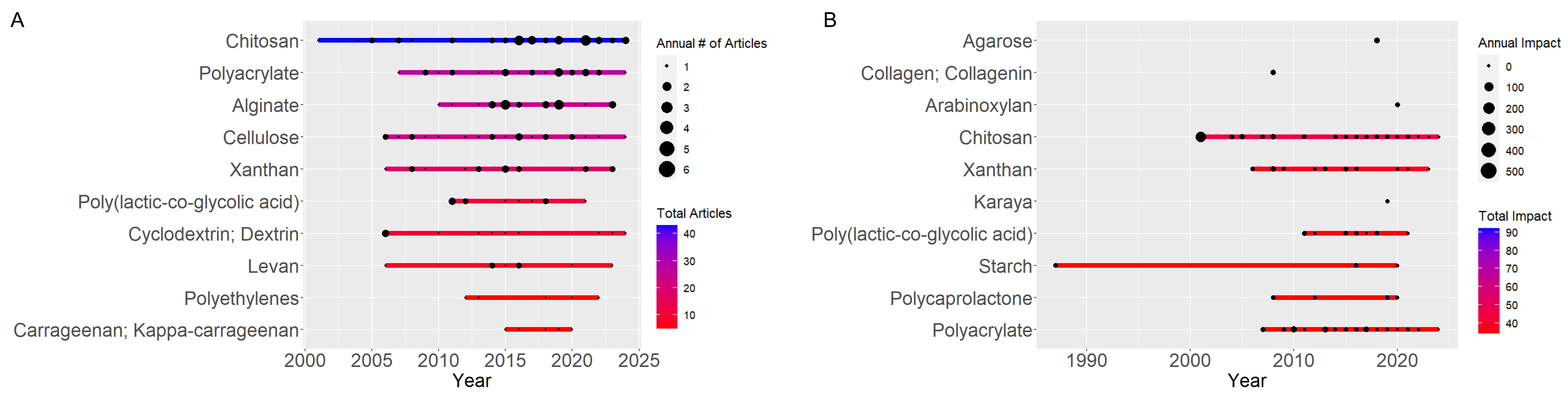
Recent trends in mannan copolymerization have highlighted a significant shift in research focus, with a notable prevalence of key materials such as Chitosan, Alginate, cellulose, and acrylic. Frequency analysis reveals that Chitosan is the most frequently studied material, followed by Alginate, cellulose, and acrylic, underscoring a strong interest in applying and developing these materials across various fields (see Figure 4).
Chitosan is the most used type of mannan due to its abundant availability from various sources, such as shrimp shells and crab shells. In addition, chitosan possesses unique properties, including high biodegradability and biocompatibility, antimicrobial activity, and the ability to easily form gels and films. Its flexible chemical structure allows for modification or combination with other polymers through crosslinking processes, resulting in nanogels with optimized properties. In nanotechnology applications, chitosan is often used to form crosslinked nanogels, which enhance physical stability, control the release of active compounds, and improve effectiveness as a drug carrier. Its mucoadhesive properties also contribute to more targeted drug delivery, making chitosan a promising candidate in the development of nanogel-based drug delivery systems.
The synergistic combination of chitosan and mannan during copolymerization further enhances the performance of nanocarriers. Chitosan, with its cationic amino groups, readily interacts with the hydroxyl and carboxyl group of mannan, facilitating the formation of stable and functional networks through hydrogen bonding, electrostatic attraction, and covalent crosslinking. Mannan contributes hydrophilicity, biodegradability, and specific targeting properties particularly its affinity for mannose receptors expressed on macrophages and cancer cells. Together, these two polysaccharides create nanogels or hydrogels with improved mechanical stability, sustained drug release behavior, and targeted delivery capacity (95). This synergy allows for greater control over drug pharmacokinetics and biodistribution, making chitosan-mannan copolymers highly attractive for advanced therapeutic applications.
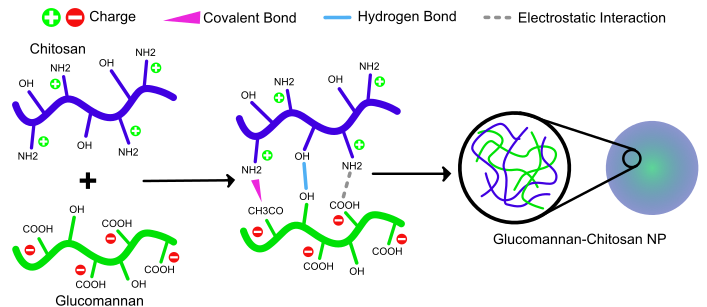
In the study by Rofeal et al. (2023), Chitosan was used for the synthesis of crosslinked nanogels (NG) loaded with caffeic acid (CafA) and eugenol (Eug). CafA and Eug have poor solubility in water, limiting their wide application in industries (96). Figure 5 illustrates the difference in the bonds formed between the main polymer components in electrostatic nanogels (non-cross-linked) and chemically crosslinked nanogels. In electrostatic nanogels, the bonds formed are only hydrogen bonds between -OH groups and electrostatic interactions between CH2COO- and NH+2.
Meanwhile, in chemically crosslinked nanogels, the same bonds are present along with an additional covalent bond CH2-CO-NH. This extra bond originates from the activation of COO- on the polymer, which is modified by a chemical crosslinker, leading to the formation of an amide bond. The study results indicate that electrostatic and chemically crosslinked nanogels exhibited colloidal stability, high blood compatibility, and in vitro serum stability. Interestingly, each NG significantly reduced the IC50 against colorectal cancer HCT-116 cells compared to conventional drugs. Based on these data, it is concluded that the studied nanogels have promising potential as candidates for functional foods and pharmaceuticals (96).
Polymers crosslink with other natural and artificial polymers, making complex crosslinked meshwork one of the critical features of hydrogels. Natural polymers offer functional groups like OH, COOH, and NH2 that are involved in forming linkages with other polymeric networks to form the architecture of the hydrogels. The inherent properties of polymers aid the crosslinking process in forming branches, linkages, and suitable crosslinkers. Two major crosslinking, i.e., physical or chemical, can be done to link polymers for hydrogel formation. Physical crosslinking occurs through the polymers' chain entanglements, hydrogen bonding, and hydrophobic interaction. Chemical crosslinking involves covalent cross-linkages between the polymer, such as epoxy compounds, glutaraldehyde, silicates, N, N' methylene bisacrylamide, etc.
Chitosan and mannan can improve drug bioavailability by passing biological barriers such as cell membranes and mucosa. Chitosan, as a cationic polymer, also aids in drug transport across cell membranes, enhancing drug penetration into cancer cells. These nanocomposites are engineered to provide controlled drug release profiles, crucial for optimizing therapeutic outcomes.
Bera et al. (2020) investigated the use of CFG-g-P(NIPA-co-MBA)-BEN nanocomposites for delivering erlotinib in lung cancer treatment, highlighting their promising drug release properties (97). The study focuses on synthesizing and characterizing carboxymethyl fenugreek galactomannan (CFG) grafted onto poly(N-isopropylacrylamide-co-N,N′-methylene-bis-acrylamide) [CFG-g-P(NIPA-co-MBA)] and bentonite (BEN) nanocomposites. Combining Chitosan and mannan in these nanocomposites improves drug bioavailability and mucoadhesive properties. This results in high drug encapsulation efficiency (93–100%) and sustained release (62–98% over 8 h). The optimized formulation enhances mucoadhesion and significantly inhibits the proliferation of A549 lung cancer cells, promoting apoptosis more effectively than the free drug. This approach aims to ensure sustained therapeutic concentrations of erlotinib within the tumor, thereby enhancing the effectiveness of lung cancer therapy. Recent research highlights the potential of combining mannan and Chitosan in drug delivery systems, demonstrating enhanced targeting and controlled release properties. For instance, Zaritzki et al. (2019) explored amphiphilic nanoparticles made from hydrolyzed galactomannan and Chitosan, effectively targeting pediatric sarcomas expressing GLUT-1 receptors. This combination enhances tumor targeting by promoting selective drug accumulation in cancer cells while minimizing damage to healthy tissues (98).
Recent studies emphasize the effectiveness of combining Chitosan and mannan derivatives in drug delivery systems, leveraging their complementary properties to enhance therapeutic outcomes. Liu et al. (2018) investigated the coacervation of Chitosan and CMKGM for colon-targeted drug delivery. They found that optimal coacervation at pH 6.5 with a 1:1 mass ratio led to stable drug carriers with improved enzyme-triggered release in the colon (99). The electrostatic interactions and hydrogen bonding between Chitosan and CMKGM provided stability in digestive fluids while allowing degradation by β-mannanase, making it suitable for targeted colon drug delivery. Further, Chitosan and CMKGM were used to develop nanogels for curcumin delivery (100). This combination showed enhanced stability under gastrointestinal conditions and sustained drug release. Similarly, Chitosan-stabilized multilayered emulsions improved curcumin bioavailability and controlled release in simulated gastrointestinal and colonic fluids (101).
In another approach, Chitosan-based Alginate hydrogels were utilized for probiotic delivery, demonstrating improved probiotic viability and targeted release across different intestinal segments. Incorporating Chitosan-stabilized emulsions in mannan hydrogel showcases the potential of combining Chitosan and mannan derivatives for advanced drug delivery systems (102). Hydrophilic polymers, such as xanthan gum, KGM, and Chitosan, were combined with semi-synthetic hydrogel (HPMC K15M) to enhance gel formation, swelling, and buoyancy in gastric fluid. This combination aimed at improving the satiety effects and weight loss potential of bupropion (103). Including KGM, degraded by β-mannanase, in the formulation with XG demonstrated potential for controlled degradation in the colon and sustained drug release (104).
The combination of Chitosan and mannan also shows promise in vaccine formulations. Chitosan enhances antigen delivery and immune response by stimulating macrophages and dendritic cells through Toll-Like Receptors (TLRs), while mannan facilitates receptor-mediated endocytosis for improved cellular uptake (105). Chitosan-mannan composite nanospheres and Chitosan-loaded Poly(lactic-co-glycolic acid) (PLGA) microparticles have demonstrated improved immunogenicity and targeted antigen delivery, with studies showing enhanced responses against avian influenza virus (AIV) antigens and anti-angiogenesis therapy (106-108). Additionally, Chitosan-mannan hydrogels have shown effective drug delivery capabilities, such as improved rifampicin encapsulation (109) and sustained release of Tenofovir in vaginal mucoadhesive tablets (110).
In a related study by Hong et al. (2008), an injectable scaffold was developed by combining collagen-coated Polylactide (PLA) microcarriers with cross-linkable Chitosan hydrogel. The collagen on the microcarriers serves as a matrix to support cell growth, while the Chitosan hydrogel provides mechanical stability and enables targeted delivery of the microcarriers within the body. The scaffold's mechanical properties were notably enhanced, and it supported effective cell growth as cells grew confluent on the microcarriers over several days. This injectable scaffold shows excellent potential for clinical applications in tissue regeneration, particularly in orthopedics, where precision and effectiveness are crucial (111).
Similarly, research highlights the promising combination of Alginate and mannan for enhancing drug delivery systems. Alginate, an anionic polymer, interacts effectively with positively charged mucosal surfaces through electrostatic bonds, while mannan, a polysaccharide with mannose residues, offers receptor-mediated endocytosis advantages. This combination has improved drug stability, release profiles, and targeting. For example, Alginate-mannan beads have been shown to enhance the stability and controlled release of drugs like Captopril, demonstrating prolonged drug action (112). The interaction between these polymers also facilitates the formation of hydrogels with improved mucosal adhesion and drug-release characteristics. Notably, pH-sensitive hydrogels incorporating Alginate and mannan, such as those functionalized with graphene oxide, exhibit effective control over drug release for agents like 5-fluorouracil, underscoring their synergistic effects in stabilizing the matrix and enhancing drug binding (113).
Additionally, the combination of Alginate with KG has shown significant potential in supersaturating drug delivery systems (SDDS), improving the solubility and stability of hydrophobic drugs like curcumin by delaying nucleation and reducing crystal growth (114). This blend effectively addresses poor solubility and unstable drug release, making it suitable for advanced drug delivery applications and targeted therapies. Alginate-mannan formulations have also demonstrated enhanced antibacterial properties and wound-healing capabilities, illustrating their potential in clinical applications (115, 116).
Bletilla striata polysaccharide (BSP), a glucomannan-based material, has been utilized to develop gastroretentive drug delivery systems. BSP's floating, swelling, and mucoadhesive properties enhance drug release profiles and strengthen mucoadhesion, crucial for prolonged gastric retention and effective drug delivery (117). Additionally, the incorporation of mannan with mucoadhesive thiolated Hydroxypropylmethylcellulose phthalate (HPMCP) microspheres has proven successful in nasal vaccine delivery, where mannan's adjuvant properties significantly boost immune responses against Actinobacillus pleuropneumoniae (118). Furthermore, mannan-decorated thiolated Eudragit microspheres have demonstrated enhanced antigen delivery to nasal-associated lymphoid tissue, eliciting strong immune responses in animal models (119).
Building on these findings, combining cellulose and mannan in drug delivery systems offers significant therapeutic advantages. Cellulose contributes mucoadhesive strength and structural stability, while mannan enhances targeted delivery to immune cells due to its affinity for CD206+ receptors on macrophages. For example, mannosylated carriers based on mannan and Polyethyleneimine (PEI), often combined with Cyclodextrin (CD), have shown selective macrophage uptake, as demonstrated in pharmacokinetic studies (120). These formulations improve antibiotic efficacy by extending drug half-life and optimizing bio-distribution, addressing critical challenges in overcoming drug resistance.
The combination of acrylic and mannan, similar to cellulose and mannan, plays a crucial role in the immune system, particularly in enhancing immune responses and drug delivery efficacy. Acrylic, like cellulose, provides the necessary structural stability and physicochemical properties. At the same time, mannan acts as an immune-targeting agent, improving interactions with immune cells such as macrophages and lymphoid tissues, thereby boosting immune responses in various therapeutic and vaccination applications. The combination of acrylic and mannan, mainly through modified galactomannan, is gaining traction in drug delivery and biomedical applications.Research highlights include the development of amphiphilic nanoparticles from hydrolyzed galactomannan and Poly(methyl methacrylate), which effectively reprogram macrophages to an anti-inflammatory phenotype, thereby enhancing wound healing (121).
Additionally, galactomannan modifications have improved drug carriers' physicochemical properties, supporting sustained release (122). Photo-curable nanocomposite hydrogels incorporating methacrylate-modified O-acetyl-galactoglucomannan and thiolated cellulose nanocrystals have demonstrated controlled release and enhanced mechanical properties (123) PLGA nanoparticles decorated with mannan and CpG ODN and PLGA microparticles for intranasal delivery of the HBsAg antigen (124, 125) demonstrate significant enhancements in systemic and mucosal immune responses. The mannan modification improves interactions with mucosal immune cells, leading to more vigorous immune responses. Additionally, mannan shows promise in nanogels designed for vaccine delivery systems, facilitating targeted antigen delivery to lymph nodes and enhancing immune responses (126). Conversely, dextrin-based carriers have been explored for allergen-specific immunotherapy, where allergen molecules are modified to reduce allergenicity while maintaining immunogenicity (127).
Based on Keyword Impact
The differences in discussion based on keyword impact and frequency highlight distinct trends in polymer usage. Polymers like chitosan, polyacrylate, alginate, cellulose, and xanthan gum are frequently used, as shown in Figure 6A. However, polymers such as Chitosan, xanthan, polyacrylate, starch, and poly(lactic-co-glycolic acid) stand out regarding keyword impact, as depicted in Figure 6B. This suggests that while some polymers are widely employed across various applications, others, though less common, have a more profound impact in specific fields like biotechnology and biomedicine, where their usage is more specialized and their effects are more significant in the relevant literature.
In addition to the Chitosan trends mentioned in the discussion in section 5.1, several other mannan combinations have been explored, such as the combination of agarose (AG), KGM, and arabinoxylan. These combinations aimed to improve the properties of pure agarose hydrogels. Pure agarose hydrogels are known for their high rigidity and brittleness, which limit their applications. The addition of KGM and arabinoxylan was investigated to observe their influence on the structure and properties of the hydrogels. Through methods such as rotational rheometry, Fourier Transform Infrared Spectroscopy, X-ray Diffraction, and Scanning Electron Microscopy, the study indicated that the flexibility of the composite hydrogels increased with higher concentrations of these additives. The synergistic interaction among KGM, arabinoxylan, and AG resulted in a more compact network structure, enhancing hydrogels' drug release capacity and loading efficiency (128, 129).
Similarly, polycaprolactone (PCL) combined with Bletilla striata polysaccharide was utilized to coat porous wafers, enhancing the release profile of Levofloxacin hydrochloride with desired floating and swelling properties. This research demonstrates that BSP-PCL can maintain controlled drug release and high mucoadhesive strength. Additionally, PCL was employed in M-hydrogel for bFGF delivery, forming a non-flowing gel at body temperature and significantly enhancing the immunogenicity of bFGF. This system also showed a controlled release of bFGF in both In Vivo and In Vitro experiments. Furthermore, the application of PCL and mannan in preparing MPCEC nanoparticles indicates the potential for enhancing immune responses through more effective vaccine delivery. In another study, using guar gum as a carrier for Rifampicin demonstrates positive interactions among PCL, guar gum, and rifampicin, with increased antibacterial activity against K. pneumoniae and S. aureus observed (130-134).
Additionally, Oxidized Konjac Glucomannan-Cassava Starch (OKGM-CS) with Sucrose Esters (SE) in developing a novel sustained-release matrix tablet via wet granulation (135). OKGM-CS, treated to reduce solubility and swelling power, was identified as a promising component for sustained-release drug formulations. SE, particularly those with an HLB value of 5, effectively reduced tablet porosity and swelling rates, thereby retarding drug release. This combination improved sustained-release performance, evident from reduced cumulative drug release and increased Mean Dissolution Time (MDT). Overall, the synergistic effect of OKGM-CS and SE demonstrates their potential as innovative agents for enhancing the sustained-release characteristics of matrix tablets, offering new avenues for pharmaceutical formulation development (134, 135).
Trends in APIs, Delivery Systems, and Targeted Diseases
Trends in APIs, delivery systems, and targeted diseases showcase a dynamic landscape in pharmaceutical and biomedical research. The discussion on APIs (Active Pharmaceutical Ingredients) often highlights advancements in molecular targeting, personalized medicine, and thedevelopment of biologics. Modern delivery systems, including nanoparticle-based carriers and liposomal formulations, are gaining prominence for enhancing drug stability, bioavailability, and targeted delivery to specific tissues or cells. These advancements are particularly pivotal in addressing complex diseases such as cancer, autoimmune disorders, and infectious diseases, where precise delivery mechanisms play a crucial role in therapeutic efficacy and minimizing adverse effects. Moreover, the emphasis on targeted diseases underscores a shift towards tailored therapies that leverage molecular insights and biomarkers to optimize treatment outcomes and patient care. As research continues to evolve, integrating these trends promises to shape the future of medicine by offering more effective and personalized approaches to disease management and treatment.
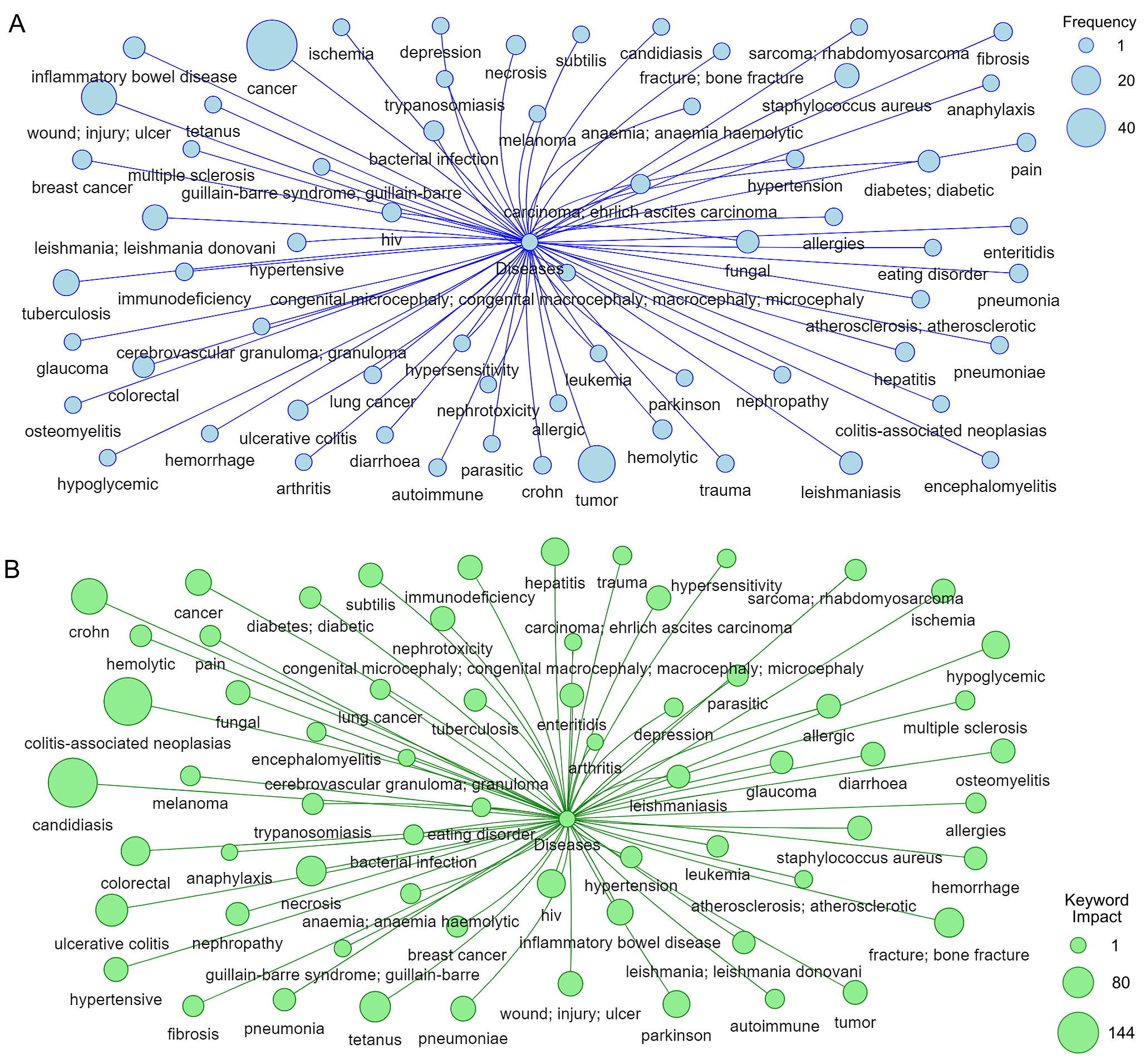
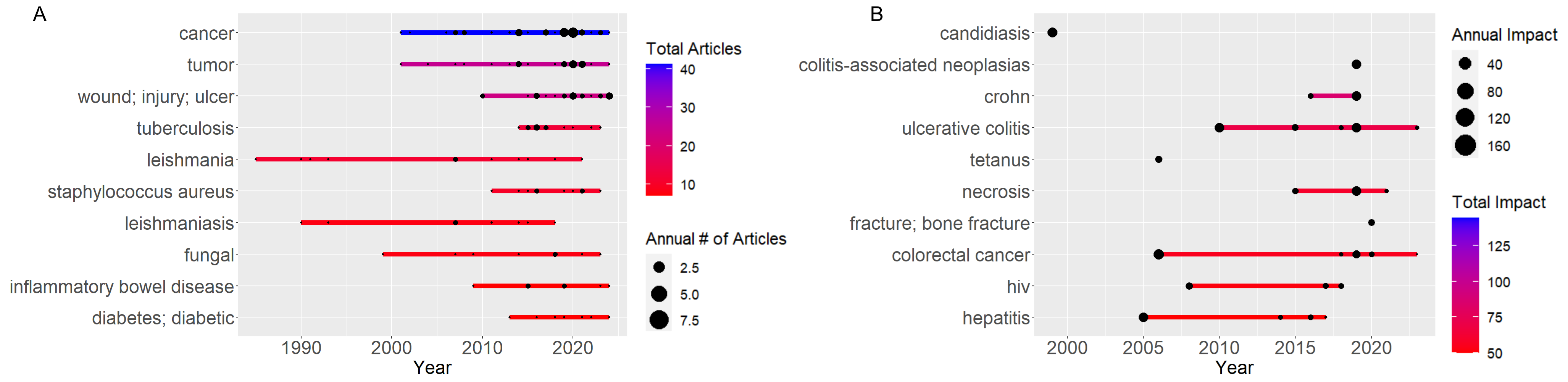
Based on Figure 7, Figures 8A, and Figure 8B, the trends in application of mannans, measured by both keyword frequency and keyword impact, indicate that mannans are most applied in cancer therapy, inflammatory bowel disease, wound healing, colitis, candidiasis, and tumor research. To statistically assess the relationship between keywords frequently and impact, a Spearman rank correlation test was conducted. This test was deemed appropriate due to the non-parametric distribution of citation data. The results (p>0.01) revealed that moderate positive correlation, suggesting that frequently mentioned keywords tend to be associated with higher citation counts. However, the data also identified several low frequency keywords such as “Cytokine storm” and “acemannan” that are linked to high impact research, indicating that emergency or niche applications may not yet be reflected by frequency alone.
Of particular interest is the significant increase in keyword frequencies related to cancer and antiinflammation therapies following the global spread of COVID-19 (See Figures 6A and 7A). This surge correlates with the emergency-driven research focus on the immunomodulatory potential of mannans, particularly in managing hyperinflammatory responses. For example, polymeric acemannan (ABPA1), isolated from Aloe vera barbadensis C(AVBEC), has shown significant inhibitory effects on cytokines storms by effectively suppressing LPS-induced proinflammatory cytokines in vitro (136).
Further in vivo studies have shown that ABPA1 treatment alleviates cytokines stroms and lung tissue damage in both LPS and influenza virus (IAV) induced mouse pneumonia models. It modulates macrophages phenotypes by enhancing M2 polarization and phagocytic activity in RAW 264 cell while suppressing LPS-induced M1 polarization. Mechanictically, ABPA2 improves mitochondrial metabolism and oxidative phosphorylation (OXPHOS) via the PI3K/Akt/GSK-3β signaling pathway. Suggesting a novel strategy for regulating macrophage activation and mitigating inflammation (137).
In parallel, nanoparticle-based delivery systems incorporating mannose moieties have shown significant promise in cancer immunotherapy. For instances, multifunctional core-crosslinked micelles form from HPMA-laurylmetacylate-hymecromone-methacrylate block copolymers have been engineered to target dendritic cells (DCs) via surface incorporation of mannose and trimannose. These system include a model peptide antigen (SIINFEKL) and lipophilic adjuvant (L18-MDP), enabling simultaneous antigen delivery and immune stimulation via tailored chemical and biological functionality.
Despite the considerable attention devoted to oncology and inflammation related conditions, several chronic or metabolic diseases remain unexplored in the context of mannan-based therapeutics. For example, diabetes mellitus, a global health concern with significant inflammatory and immune components presents a promising yet relatively untapped domain for mannan research. Recent studies have suggested that certain mannans, particularly immunomodulatory variants such as acemannan, may have the capacity to modulate insulin sensitivity, glycemic control, and islet cell inflammation, yet systematic investigations in this area remain scarce.
Micelles without carbohydrate units show minimal binding to DCs, while mannose and tri-mannose functionalization significantly improves this binding. Flow cytometric analysis and mannan blocking studies reveal that effective micelle binding requires mannose receptors and DC-SIGN on the DCs. This binding can be inhibited by blocking with mannan. Micelles loaded with adjuvants and functionalized with mannose and trimannose not only activate DCs but also stimulate DCs pre-incubated with antigen-conjugated micelles to induce the proliferation of antigen-specific CD8+ T cells, thereby enhancing the immune response against tumors (138). A study about recombinant adenovirus vector modified with mannan was used to deliver VE-cad (AdVEC-m) and to explore its feasibility as an antitumor agent in mouse cancer models. Angiogenesis inhibitors have been tested extensively and are recognized as valuable agents for cancer therapy. The complex structures of endothelial cell-to-cell junctions formed by different adhesion molecules provide hopeful molecular targets for anti-angiogenic treatment. Monoclonal antibodies (mAbs) against VE-cad inhibited tumor growth and metastasis in various mouse models. Current vaccine strategies that can deliver antigens to the immune system effectively have been developed using mannan conjugated antigens. Mannose receptors (MRs, CD206), a new family of multilectin receptor proteins, are the most ubiquitous receptors expressed on Antigen-presenting cells (APCs), including dendritic cells (DCs) and macrophage cells. The potent recognition of receptors with mannan (poly-mannose derived from the active component of zymosan) has been targeted with ligands to deliver glycosylated antigen peptides or proteins into APCs. In addition, mannan receptor-mediated gene transfer has also been tested for cancer immunotherapy. It was reported that oxidized and reduced mannan delivery was superior to DNA alone protection of mice from tumors, resulting in enhanced antigen uptake and presentation and efficient immune responses to antigens (139).
Based on impact keywords Figure 8B, mannans are most frequently used in Crohn's disease, Candidiasis, and Colitis studies. Macrophages play essential roles in the pathophysiology of numerous disorders. Macrophages form an important line of defense against bacterial and viral infection but have detrimental functions in chronic inflammatory diseases such as bowel disease, rheumatoid arthritis (RA), and multiple sclerosis, as well as in metabolism disease, atherosclerosis, and cancer. Macrophages are well-known therapeutic targets for various diseases. Recently, tylophorine maleate (NK007), a small molecule compound, was found to have an extraordinary inhibitory activity against TNF alpha production, this downing it with a great potential for the treatment of Inflammatory Bowel Disease (IBD) and considering the high cost and inconvenient administration routes of biological agents (140). Treating diseases involving macrophages, particularly chronic medication, has practical importance for clinical application, such as improving drug selectivity to reduce off-target effects through chemical modification, and the targeted delivery of drug molecules to macrophages is a practical alternative. Once the drug molecules are encapsulated into carrier systems, the therapeutic agent needed for a clinical effect may be reduced, potentially reducing drug-induced toxicity and other side effects. The use of Glucan mannan particles (GMPs) was explored to create a potential carrier system for delivering the small molecule NK007 to macrophages for treating inflammatory diseases. This formulation employed Chitosan, Tripolyphosphate (TPP), and Alginate to form colloidal particles with the model drug NK007 through electrostatic interactions, tightly incorporating NK007 within the GMPs. The NK007-encapsulated GMPs (GMP-NK007) effectively delivered NK007 to macrophages In Vitro and In Vivo. A dextran sulfate sodium (DSS)-induced murine colitis model, mimicking human ulcerative colitis, was utilized to assess the effects of the formed particles administered orally, highlighting the utility of this system as an efficient oral delivery system targeted towards macrophages (140, 141).
Author Perspective
Exploring trends in mannan-based drug delivery systems through bibliometric analysis and natural language processing (NLP) underscores the increasing importance of mannan as a versatile polymer in therapeutic applications. This study reveals a significant alignment between emerging trends and current research focusing on biocompatible materials that enhance drug efficacy while mitigating adverse effects. Despite this progress, inconsistencies in understanding the biological interactions of mannan highlight a critical gap that necessitates further investigation into its pharmacokinetics and biodistribution.
The findings indicate that while combining mannan with other polymers often yields improved drug delivery systems, there is a noticeable scarcity of research directed toward therapeutic areas outside of cancer and inflammatory diseases. This oversight suggests untapped potential for mannan in diverse applications, particularly within advanced drug delivery technologies such as nanoparticles. Notably, integrating machine learning algorithms could facilitate the optimization of formulations by predicting interactions and enhancing system design.
Looking ahead, it is crucial for future studies to address existing methodological limitations, focusing on standardized protocols and robust experimental designs to ensure replicability. The implications of these findings are poised to transform pharmaceutical practices, with mannan-based systems aligning with the principles of personalized medicine. By enhancing targeted delivery strategies, these innovations could significantly improve patient outcomes in treating complex diseases. However, a critical evaluation of the current research landscape reveals the need for more extensive clinical validation and translational studies to solidify mannan's role in drug delivery systems. As the field progresses, advancements in biopolymer science will likely lead to innovative solutions that enhance therapeutic efficacy.
Conclusion
In conclusion, investigating mannan-based drug delivery systems reveals significant trends and key findings that underscore their potential in pharmaceutical applications. The bibliometric and NLP analyses highlight Chitosan, Alginate, and polycaprolactone as frequently used polymers, while agarose and collagen exhibit notable keyword impact, suggesting specialized applications in biotechnology and biomedicine. While promising, few mannan systems have reach clinical stages, acemannan-based wound products being a rare example indicating a gap between preclinical innovation and clinical translation. Limitations of this study include potential NLP biases, inconsistent terminology, and challenges in scalability due to manual validation. Looking forward, integrating machine learning for predicting polymer ratios, optimizing formulation and stimulating drug release can accelerate the design of smart, personalized nanocarriers and support the clinical advancement of mannan-based therapeutics.
Declarations
Ethics Statement
Not relevant
Data Availability
Not applicable.
Funding Information
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflicting interest.
References
- Visan AI, Cristescu R. Polysaccharide-Based Coatings as Drug Delivery Systems. Pharmaceutics. 2023 Aug 29;15(9):2227.
- Jabeen N, Atif M. Polysaccharides based biopolymers for biomedical applications: A review. Polym Adv Technol. 2024 Jan 9;35(1).
- Picos-Corrales LA, Morales-Burgos AM, Ruelas-Leyva JP, Crini G, García-Armenta E, Jimenez-Lam SA, et al. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers (Basel). 2023 Jan 19;15(3):526.
- Pratiwi RD, El Muttaqien S, Gustini N, Difa NS, Syahputra G, Rosyidah A. Eco-friendly synthesis of chitosan and its medical application: from chitin extraction to nanoparticle preparation. ADMET DMPK. 2023 Sep 23;
- Yi K, Miao S, Yang B, Li S, Lu Y. Harnessing the Potential of Chitosan and Its Derivatives for Enhanced Functionalities in Food Applications. Foods. 2024 Jan 29;13(3):439.
- Ojima T. Polysaccharide-degrading enzymes from herbivorous marine invertebrates. In: Marine Enzymes for Biocatalysis. Elsevier; 2013. p. 333–371.
- Moreira LRS, Filho EXF. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol [Internet]. 2008 May 1;79(2):165–78.
- Liu M, Fan J, Wang K, He Z. Synthesis, Characterization, and Evaluation of Phosphated Cross-Linked Konjac Glucomannan Hydrogels for Colon-Targeted Drug Delivery. Drug Deliv. 2007 Jan 10;14(6):397–402.
- Alvarez-Manceñido F, Braeckmans K, De Smedt SC, Demeester J, Landin M, Martínez-Pacheco R. Characterization of diffusion of macromolecules in konjac glucomannan solutions and gels by fluorescence recovery after photobleaching technique. Int J Pharm. 2006 Jun;316(1–2):37–46.
- Ai T, Shang L, He C, Teng Y, Ren C, Zhou P, et al. Development of multi-layered gastric floating tablets based on konjac glucomannan: a modified calcium supplement with enhanced bioavailability. Food Funct. 2019;10(10):6429–6437.
- Xu M, Huang J, Jiang S, He J, Wang Z, Qin H, et al. Glucose sensitive konjac glucomannan/concanavalin A nanoparticles as oral insulin delivery system. Int J Biol Macromol. 2022 Mar;202:296–308.
- Sheng K, Pouniotis DS, Wright MD, Tang CK, Lazoura E, Pietersz GA, et al. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006 Jul 26;118(3):372–383.
- Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit ECM, Lanzavecchia A, et al. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997 Sep;27(9):2417–2425.
- Xu Y, Ma S, Zhao J, Chen H, Si X, Huang Z, et al. Mannan-decorated pathogen-like polymeric nanoparticles as nanovaccine carriers for eliciting superior anticancer immunity. Biomaterials. 2022 May;284:121489.
- Liu L, Zhao J, Huang Z, Xu Y, Chen H, Qiao R, et al. Mannan-decorated STING-activating vaccine carrier for spatial coordinative stimulating antigen-specific immune responses. Fundamental Research. 2023 May;
- Brito ACM, Oliveira MCF, Oliveira ON, Silva FN, Amancio DR. Network Analysis and Natural Language Processing to Obtain a Landscape of the Scientific Literature on Materials Applications. ACS Appl Mater Interfaces. 2023 Jun 14;15(23):27437–27446.
- Brito ACM, Oliveira MCF, Oliveira ON, Silva FN, Amancio DR. History of Chemistry of Materials According to Topic Evolution Based on Network Analysis and Natural Language Processing. Chemistry of Materials. 2024 Jan 9;36(1):1–7.
- Neumann M, King D, Beltagy I, Ammar W. ScispaCy: Fast and Robust Models for Biomedical Natural Language Processing. In: Proceedings of the 18th BioNLP Workshop and Shared Task. Stroudsburg, PA, USA: Association for Computational Linguistics; 2019. p. 319–327.
- Hu C, Gong H, He Y. Data driven identification of international cutting edge science and technologies using SpaCy. Margherita A, editor. PLoS One. 2022 Oct 12;17(10):e0275872.
- Campbell JC, Hindle A, Stroulia E. Latent Dirichlet Allocation: Extracting Topics from Software Engineering Data. The Art and Science of Analyzing Software Data. 2015;3:139–159.
- Farkhod A, Abdusalomov A, Makhmudov F, Cho YI. LDA-Based Topic Modeling Sentiment Analysis Using Topic/Document/Sentence (TDS) Model. Applied Sciences. 2021 Nov 23;11(23):11091.
- Baek KR, Rani Ramakrishnan S, Kim SJ, Seo SO. Yeast cell wall mannan structural features, biological activities, and production strategies. Vol. 10, Heliyon. Elsevier Ltd; 2024.
- Hlalukana N, Magengelele M, Malgas S, Pletschke BI. Enzymatic conversion of mannan-rich plant waste biomass into prebiotic mannooligosaccharides. Vol. 10, Foods. MDPI; 2021.
- Capek P, Kubačková M, Alföldi J, Bilisics L, Lišková D, Kákoniová D. Galactoglucomannan from the secondary cell wall of Picea abies L. Karst. Carbohydr Res. 2000 Nov;329(3):635–645.
- Guerreiro F, Swedrowska M, Patel R, Flórez-Fernández N, Torres MD, Rosa da Costa AM, et al. Engineering of konjac glucomannan into respirable microparticles for delivery of antitubercular drugs. Int J Pharm. 2021 Jul;604:120731.
- Guerreiro F, Pontes JF, Rosa da Costa AM, Grenha A. Spray-drying of konjac glucomannan to produce microparticles for an application as antitubercular drug carriers. Powder Technol. 2019 Jan;342:246–252.
- Guerreiro F, Swedrowska M, Patel R, Flórez-Fernández N, Torres MD, Rosa da Costa AM, et al. Engineering of konjac glucomannan into respirable microparticles for delivery of antitubercular drugs. Int J Pharm. 2021 Jul;604:120731.
- Guerreiro F, Pontes JF, Gaspar MM, Rosa da Costa AM, Faleiro ML, Grenha A. Respirable konjac glucomannan microparticles as antitubercular drug carriers: Effects of in vitro and in vivo interactions. Int J Biol Macromol. 2023 Sep;248:125838.
- Dong L, Xia S, Luo Y, Diao H, Zhang J, Chen J, et al. Targeting delivery oligonucleotide into macrophages by cationic polysaccharide from Bletilla striata successfully inhibited the expression of TNF-α. Journal of Controlled Release. 2009 Mar;134(3):214–220.
- Meng FB, Zhang Q, Li YC, Li JJ, Liu DY, Peng LX. Konjac glucomannan octenyl succinate as a novel encapsulation wall material to improve curcumin stability and bioavailability. Carbohydr Polym. 2020 Jun;238:116193.
- Zhang Q, Fu H, Zhang Y, Li L, Yan G. Rapidly degradable konjac glucomannan hydrogels cross-linked with olsalazine for colonic drug release. Biomed Mater Eng. 2024 Mar 8;35(2):125–137.
- Liu M, Fan J, Wang K, He Z. Synthesis, Characterization, and Evaluation of Phosphated Cross-Linked Konjac Glucomannan Hydrogels for Colon-Targeted Drug Delivery. Drug Deliv. 2007 Jan 10;14(6):397–402.
- Ai T, Shang L, He C, Teng Y, Ren C, Zhou P, et al. Development of multi-layered gastric floating tablets based on konjac glucomannan: a modified calcium supplement with enhanced bioavailability. Food Funct. 2019;10(10):6429–6437.
- Josino MAA, Rocha da Silva C, de Andrade Neto JB, Barroso FDD, Juvêncio da Silva L, Cavalcanti BC, et al. Development and in vitro evaluation of microparticles of fluoxetine in galactomannan against biofilms of S. aureus methicilin resistant. Carbohydr Polym. 2021 Jan;252:117184.
- R S, M Joseph M, Sen A, K RP, BS U, TT S. Galactomannan armed superparamagnetic iron oxide nanoparticles as a folate receptor targeted multi-functional theranostic agent in the management of cancer. Int J Biol Macromol. 2022 Oct;219:740–753.
- Bakhshy E, Zarinkamar F, Nazari M. Isolation, qualitative and quantitative evaluation of galactomannan during germination of Trigonella persica (Fabaceae) seed. Int J Biol Macromol. 2019 Sep;137:286–295.
- Ogunjimi AT, Melo SMG, Vargas-Rechia CG, Emery FS, Lopez RFV. Hydrophilic polymeric nanoparticles prepared from Delonix galactomannan with low cytotoxicity for ocular drug delivery. Carbohydr Polym. 2017 Feb;157:1065–1075.
- Campia P, Ponzini E, Rossi B, Farris S, Silvetti T, Merlini L, et al. “Aerogels of enzymatically oxidized galactomannans from leguminous plants: Versatile delivery systems of antimicrobial peptides and enzymes.” Carbohydr Polym. 2017 Feb;158:102–111.
- Pawar HA, Lalitha KG. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int J Biol Macromol. 2014 Apr;65:167–175.
- Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X, et al. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020 Sep 19;9(1).
- Ruan GX, Chen YZ, Yao XL, Du A, Tang GP, Shen YQ, et al. Macrophage mannose receptor-specific gene delivery vehicle for macrophage engineering. Acta Biomater. 2014 May;10(5):1847–1855.
- Ruan GX, Zhang TY, Li LM, Zhang XG, Shen YQ, Tabata Y, et al. Hepatic-Targeted Gene Delivery Using Cationic Mannan Vehicle. Mol Pharm. 2014 Oct 6;11(10):3322–3329.
- Jo J ichiro, Okazaki A, Nagane K, Yamamoto M, Tabata Y. Preparation of Cationized Polysaccharides as Gene Transfection Carrier for Bone Marrow-Derived Mesenchymal Stem Cells. J Biomater Sci Polym Ed. 2010 Jan 2;21(2):185–204.
- Dong L, Xia S, Luo Y, Diao H, Zhang J, Chen J, et al. Targeting delivery oligonucleotide into macrophages by cationic polysaccharide from Bletilla striata successfully inhibited the expression of TNF-α. Journal of Controlled Release. 2009 Mar;134(3):214–220.
- Campia P, Ponzini E, Rossi B, Farris S, Silvetti T, Merlini L, et al. “Aerogels of enzymatically oxidized galactomannans from leguminous plants: Versatile delivery systems of antimicrobial peptides and enzymes.” Carbohydr Polym. 2017 Feb;158:102–111.
- Chen X, Wang S, Lu M, Chen Y, Zhao L, Li W, et al. Formation and Characterization of Light-Responsive TEMPO-Oxidized Konjac Glucomannan Microspheres. Biomacromolecules. 2014 Jun 9;15(6):2166–2171.
- Ganie SA, Naik RA, Ali A, Mir TA, Mazumdar N. Preparation, characterization, release and antianemic studies of guar gum functionalized Iron complexes. Int J Biol Macromol. 2021 Jul;183:1495–1504.
- Lima LRM, Ramos EL de L, Silva MFS, Ribeiro F de OS, Sousa JS, Pessoa C, et al. Poly(N-isopropylacrylamide)/galactomannan from Delonix regia seed thermal responsive graft copolymer via Schiff base reaction. Int J Biol Macromol. 2021 Jan;166:144–154.
- Francis AP, Jayakrishnan A. Conjugating doxorubicin to polymannose: a new strategy for target specific delivery to lung cancer cells. J Biomater Sci Polym Ed. 2019 Nov 2;30(16):1471–1488.
- Zhao R, Du S, Liu Y, Lv C, Song Y, Chen X, et al. Mucoadhesive-to-penetrating controllable peptosomes-in-microspheres co-loaded with anti-miR-31 oligonucleotide and Curcumin for targeted colorectal cancer therapy. Theranostics. 2020;10(8):3594–3611.
- Tang CK, Lodding J, Minigo G, Pouniotis DS, Plebanski M, Scholzen A, et al. Mannan‐mediated gene delivery for cancer immunotherapy. Immunology. 2007 Mar 20;120(3):325–335.
- Tang CK, Sheng KC, Esparon SE, Proudfoot O, Apostolopoulos V, Pietersz GA. Molecular basis of improved immunogenicity in DNA vaccination mediated by a mannan based carrier. Biomaterials. 2009 Mar;30(7):1389–1400.
- Zhang J, Wang Y, Wu Y, Ding ZY, Luo XM, Zhong WN, et al. Mannan-modified adenovirus encoding VEGFR-2 as a vaccine to induce anti-tumor immunity. J Cancer Res Clin Oncol. 2014 May 14;140(5):701–712.
- Tang CK, Sheng KC, Pouniotis D, Esparon S, Son HY, Kim CW, et al. Oxidized and reduced mannan mediated MUC1 DNA immunization induce effective anti-tumor responses. Vaccine. 2008 Jul;26(31):3827–3834.
- Xu S, Yan S, You J, Wu X. Antibacterial Micelles-Loaded Carboxymethyl Chitosan/Oxidized Konjac Glucomannan Composite Hydrogels for Enhanced Wound Repairing. ACS Appl Mater Interfaces. 2024 Mar 20;16(11):13563–23572.
- Zong Q, Peng X, Wu H, Ding Y, Ye X, Gao X, et al. Copper-gallate metal-organic framework encapsulated multifunctional konjac glucomannan microneedles patches for promoting wound healing. Int J Biol Macromol. 2024 Feb;257:128581.
- Bera H, Abbasi YF, Gajbhiye V, Liew KF, Kumar P, Tambe P, et al. Carboxymethyl fenugreek galactomannan-g-poly(N-isopropylacrylamide-co-N,N′-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Materials Science and Engineering: C. 2020 May;110:110628.
- Verma S, Rimpy, Ahuja M. Carboxymethyl modification of Cassia obtusifolia galactomannan and its evaluation as sustained release carrier. Int J Biol Macromol. 2020 Dec;164:3823–3834.
- Rofeal M, Abdelmalek F, Pietrasik J, Steinbüchel A. A comparative study between two carboxymethylated polysaccharides/protein electrostatic and cross-linked nanogels constructed for caffeic acid and eugenol delivery. Int J Biol Macromol. 2023 Aug;245:125585.
- Wu C, Sun J, Jiang H, Li Y, Pang J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021 Nov;362:130242.
- Zhu C, Zhang X, Gan J, Geng D, Bian X, Cheng Y, et al. A pH-sensitive hydrogel based on carboxymethylated konjac glucomannan crosslinked by sodium trimetaphosphate: Synthesis, characterization, swelling behavior and controlled drug release. Int J Biol Macromol. 2023 Mar;232:123392.
- Rofeal M, Abdelmalek F, Pietrasik J, Steinbüchel A. A comparative study between two carboxymethylated polysaccharides/protein electrostatic and cross-linked nanogels constructed for caffeic acid and eugenol delivery. Int J Biol Macromol. 2023 Aug;245:125585.
- Shi C, Zhu P, Chen N, Ye X, Wang Y, Xiao S. Preparation and sustainable release of modified konjac glucomannan/chitosan nanospheres. Int J Biol Macromol. 2016 Oct;91:609–614.
- Tang W, Wei Y, Lu W, Chen D, Ye Q, Zhang C, et al. Fabrication, characterization of carboxymethyl konjac glucomannan/ovalbumin-naringin nanoparticles with improving in vitro bioaccessibility. Food Chem X. 2022 Dec;16:100477.
- Zhu C, Zhang X, Gan J, Geng D, Bian X, Cheng Y, et al. A pH-sensitive hydrogel based on carboxymethylated konjac glucomannan crosslinked by sodium trimetaphosphate: Synthesis, characterization, swelling behavior and controlled drug release. Int J Biol Macromol. 2023 Mar;232:123392.
- Wu C, Sun J, Jiang H, Li Y, Pang J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021 Nov;362:130242.
- Xiao JX, Wang LH, Xu TC, Huang GQ. Complex coacervation of carboxymethyl konjac glucomannan and chitosan and coacervate characterization. Int J Biol Macromol. 2019 Feb;123:436–445.
- Mittal N, Kaur G. Leucaena leucocephala (Lam.) galactomannan nanoparticles: Optimization and characterization for ocular delivery in glaucoma treatment. Int J Biol Macromol. 2019 Oct;139:1252–1262.
- Shi C, Zhu P, Chen N, Ye X, Wang Y, Xiao S. Preparation and sustainable release of modified konjac glucomannan/chitosan nanospheres. Int J Biol Macromol. 2016 Oct;91:609–614.
- Bera H, Mothe S, Maiti S, Vanga S. Carboxymethyl fenugreek galactomannan-gellan gum-calcium silicate composite beads for glimepiride delivery. Int J Biol Macromol. 2018 Feb;107:604–614.
- Gupta S, Vyas SP. Development and characterization of amphotericin B bearing emulsomes for passive and active macrophage targeting. J Drug Target. 2007 Jan 8;15(3):206–217.
- Gupta S, Dube A, Vyas SP. Antileishmanial efficacy of amphotericin B bearing emulsomes against experimental visceral leishmaniasis. J Drug Target. 2007 Jan 8;15(6):437–444.
- Vyas SP, Khatri K, Goyal AK. Functionalized nanocarrier(s) to image and target fungi infected immune cells. Med Mycol. 2009 Jan;47(s1):S362–S368.
- Jain S, Vyas SP. Mannosylated Niosomes as Adjuvant-Carrier System for Oral Mucosal Immunization. J Liposome Res. 2006 Jan 9;16(4):331–345.
- Jain S, Singh P, Mishra V, Vyas SP. Mannosylated niosomes as adjuvant–carrier system for oral genetic immunization against Hepatitis B. Immunol Lett. 2005 Oct;101(1):41–49.
- Vyas SP, Katare YK, Mishra V, Sihorkar V. Ligand directed macrophage targeting of amphotericin B loaded liposomes. Int J Pharm. 2000 Dec;210(1–2):1–14.
- Gupta S, Dube A, Vyas SP. Antileishmanial efficacy of amphotericin B bearing emulsomes against experimental visceral leishmaniasis. J Drug Target. 2007 Jan 8;15(6):437–444.
- Vyas SP, Khatri K, Goyal AK. Functionalized nanocarrier(s) to image and target fungi infected immune cells. Med Mycol. 2009 Jan;47(s1):S362–S368.
- Jain S, Vyas SP. Mannosylated Niosomes as Adjuvant-Carrier System for Oral Mucosal Immunization. J Liposome Res. 2006 Jan 9;16(4):331–345.
- Gupta S, Vyas SP. Development and characterization of amphotericin B bearing emulsomes for passive and active macrophage targeting. J Drug Target. 2007 Jan 8;15(3):206–217.
- Ahmadpour S, Hosseinimehr SJ. PASylation as a Powerful Technology for Improving the Pharmacokinetic Properties of Biopharmaceuticals. Curr Drug Deliv [Internet]. 2018 Mar 27;15(3):331–341.
- Paurević M, Šrajer Gajdošik M, Ribić R. Mannose Ligands for Mannose Receptor Targeting. Int J Mol Sci [Internet]. 2024 Jan 23;25(3):1370.
- Li Z, Gao X, Yan X, Deng Y, Ma H. PEGylated nanoemulsions containing 1,2-distearoyl-sn-glycero-3-phosphoglycerol induced weakened accelerated blood clearance phenomenon. Drug Deliv Transl Res [Internet]. 2022 Oct 29;12(10):2569–2579.
- Reis AC, dos Santos L V., Santos KR, Lima-Tenório MK, Paludo KS, Maurício MR, et al. Chemically crosslinked guar gum hydrogels: An investigation on the water transport and its relationship with hydrocortisone release. Int J Pharm. 2022 Apr;617:121626.
- Mahto A, Mishra S. Design, development and validation of guar gum based pH sensitive drug delivery carrier via graft copolymerization reaction using microwave irradiations. Int J Biol Macromol. 2019 Oct;138:278–291.
- An K, Kang H, Zhang L, Guan L, Tian D. Preparation and properties of thermosensitive molecularly imprinted polymer based on konjac glucomannan and its controlled recognition and delivery of 5-fluorouracil. J Drug Deliv Sci Technol. 2020 Dec;60:101977.
- Wang Q, Xu W, Koppolu R, van Bochove B, Seppälä J, Hupa L, et al. Injectable thiol-ene hydrogel of galactoglucomannan and cellulose nanocrystals in delivery of therapeutic inorganic ions with embedded bioactive glass nanoparticles. Carbohydr Polym. 2022 Jan;276:118780.
- Ferreira SA, Pereira P, Sampaio P, Coutinho PJG, Gama FM. Supramolecular assembled nanogel made of mannan. J Colloid Interface Sci. 2011 Sep;361(1):97–108.
- Rodrigues LC, Fernandes EM, Ribeiro AR, Ribeiro AP, Silva SS, Reis RL. Physicochemical features assessment of acemannan-based ternary blended films for biomedical purposes. Carbohydr Polym. 2021 Apr;257:117601.
- Li L, Xu W, Luo Y, Lao C, Tong X, Du J, et al. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr Polym. 2022 Dec;297:120032.
- Ahn Y, Song Y, Kim H, Kwak SY. Formation of cellulose-carbene complex via depolymerization in ILs: Dependence of IL types on kinetics, conformation and dispersity. Carbohydr Polym. 2017 Mar;159:86–93.
- Khan S, Khan NA, Bano B. In-sights into the effect of heavy metal stress on the endogenous mustard cystatin. Int J Biol Macromol. 2017 Dec;105:1138–1147.
- Song J, Li Q, Dzakpasu M, Wang XC, Chang N. Integrating stereo-elastic packing into ecological floating bed for enhanced denitrification in landscape water. Bioresour Technol. 2020 Mar;299:122601.
- Bohutskyi P, Kucek LA, Hill E, Pinchuk GE, Mundree SG, Beliaev AS. Conversion of stranded waste-stream carbon and nutrients into value-added products via metabolically coupled binary heterotroph-photoautotroph system. Bioresour Technol. 2018 Jul;260:68–75.
- Kumar D, Gihar S, Shrivash MK, Kumar P, Kundu PP. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int J Biol Macromol [Internet]. 2020 Nov;163:2097–2112.
- Rofeal M, Abdelmalek F, Pietrasik J, Steinbüchel A. A comparative study between two carboxymethylated polysaccharides/protein electrostatic and cross-linked nanogels constructed for caffeic acid and eugenol delivery. Int J Biol Macromol. 2023 Aug;245:125585.
- Bera H, Abbasi YF, Gajbhiye V, Liew KF, Kumar P, Tambe P, et al. Carboxymethyl fenugreek galactomannan-g-poly(N-isopropylacrylamide-co-N,N′-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Materials Science and Engineering: C. 2020 May;110:110628.
- Zaritski A, Castillo-Ecija H, Kumarasamy M, Peled E, Sverdlov Arzi R, Carcaboso ÁM, et al. Selective Accumulation of Galactomannan Amphiphilic Nanomaterials in Pediatric Solid Tumor Xenografts Correlates with GLUT1 Gene Expression. ACS Appl Mater Interfaces. 2019 Oct 23;11(42):38483–38496.
- Xiao JX, Wang LH, Xu TC, Huang GQ. Complex coacervation of carboxymethyl konjac glucomannan and chitosan and coacervate characterization. Int J Biol Macromol. 2019 Feb;123:436–445.
- Wu C, Sun J, Jiang H, Li Y, Pang J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021 Nov;362:130242.
- Wang LH, Xiao JX, Li XD, Huang GQ. Carboxymethyl konjac glucomannan coating on multilayered emulsions for improved bioavailability and targeted delivery of curcumin. Food Funct. 2021;12(12):5429–39.
- Ding X, Li D, Xu Y, Wang Y, Liang S, Xie L, et al. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized emulsions incorporated into alginate as microcapsule matrix for intestinal-targeted delivery of probiotics: In vivo and in vitro studies. Int J Biol Macromol. 2023 Dec;253:126931.
- Teaima M, Abdel Hamid MM, Shoman NA, Jasti BR, El-Nabarawi MA. <p>Promising Swellable Floating Bupropion Tablets: Formulation, in vitro/in vivo Evaluation and Comparative Pharmacokinetic Study in Human Volunteers</p>. Drug Des Devel Ther. 2020 Jul;Volume 14:2741–57.
- Alvarez-Manceñido F, Landin M, Martínez-Pacheco R. Konjac glucomannan/xanthan gum enzyme sensitive binary mixtures for colonic drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2008 Jun;69(2):573–581.
- Shi C, Zhu P, Chen N, Ye X, Wang Y, Xiao S. Preparation and sustainable release of modified konjac glucomannan/chitosan nanospheres. Int J Biol Macromol. 2016 Oct;91:609–614.
- Dai X, He J, Zhang R, Wu G, Xiong F, Zhao B. Co-delivery of polyinosinic:polycytidylic acid and flagellin by poly(lactic-<em>co</em>-glycolic acid) MPs synergistically enhances immune response elicited by intranasally delivered hepatitis B surface antigen. Int J Nanomedicine. 2017 Sep;Volume 12:6617–6632.
- Alkie TN, Yitbarek A, Taha-Abdelaziz K, Astill J, Sharif S. Characterization of immunogenicity of avian influenza antigens encapsulated in PLGA nanoparticles following mucosal and subcutaneous delivery in chickens. PLoS One. 2018 Nov 1;13(11):e0206324.
- Zhao Z, Yao Y, Ding Z, Chen X, Xie K, Luo Y, et al. Antitumour immunity mediated by mannan-modified adenovirus vectors expressing VE-cadherin. Vaccine. 2011 Jun;29(25):4218–4224.
- Yuan X, Amarnath Praphakar R, Munusamy MA, Alarfaj AA, Suresh Kumar S, Rajan M. Mucoadhesive guargum hydrogel inter-connected chitosan-g-polycaprolactone micelles for rifampicin delivery. Carbohydr Polym. 2019 Feb;206:1–10.
- Notario-Pérez F, Martín-Illana A, Cazorla-Luna R, Ruiz-Caro R, Bedoya LM, Tamayo A, et al. Influence of Chitosan Swelling Behaviour on Controlled Release of Tenofovir from Mucoadhesive Vaginal Systems for Prevention of Sexual Transmission of HIV. Mar Drugs. 2017 Feb 21;15(2):50.
- Hong Y, Gong Y, Gao C, Shen J. Collagen‐coated polylactide microcarriers/chitosan hydrogel composite: Injectable scaffold for cartilage regeneration. J Biomed Mater Res A. 2008 Jun 5;85A(3):628–637.
- Pawar HA, Lalitha KG, Ruckmani K. Alginate beads of Captopril using galactomannan containing Senna tora gum, guar gum and locust bean gum. Int J Biol Macromol. 2015 May;76:119–131.
- Wang J, Liu C, Shuai Y, Cui X, Nie L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf B Biointerfaces. 2014 Jan;113:223–229.
- Tian B, Li L, Kang K, Peng D, Shi Y, Wang P. Crystallization inhibitory effects of konjac glucomannan, sodium alginate and xanthan gum on curcumin in supersaturated solution. Int J Biol Macromol. 2023 Aug;245:125489.
- Guo Y, Xie B, Jiang M, Yuan L, Jiang X, Li S, et al. Facile and eco-friendly fabrication of biocompatible hydrogel containing CuS@Ser NPs with mechanical flexibility and photothermal antibacterial activity to promote infected wound healing. J Nanobiotechnology. 2023 Aug 10;21(1):266.
- Koop HS, Da-lozzo EJ, de Freitas RA, Franco CRC, Mitchell DA, Silveira JLM. Rheological Characterization of a Xanthan–Galactomannan Hydrogel Loaded with Lipophilic Substances. J Pharm Sci. 2012 Jul;101(7):2457–2467.
- Li Z, Zeng R, Yang L, Ren X, Maffucci KG, Qu Y. Development and Characterization of PCL Electrospun Membrane-Coated Bletilla striata Polysaccharide-Based Gastroretentive Drug Delivery System. AAPS PharmSciTech. 2020 Feb 13;21(2):66.
- Li HS, Shin MK, Singh B, Maharjan S, Park TE, Kang SK, et al. Nasal immunization with mannan-decorated mucoadhesive HPMCP microspheres containing ApxIIA toxin induces protective immunity against challenge infection with Actinobacillus pleuropneumoiae in mice. Journal of Controlled Release. 2016 Jul;233:114–125.
- Li HS, Singh B, Park TE, Hong ZS, Kang SK, Cho CS, et al. Mannan-decorated thiolated Eudragit microspheres for targeting antigen presenting cells via nasal vaccination. European Journal of Pharmaceutical Sciences. 2015 Dec;80:16–25.
- Zlotnikov ID, Vigovskiy MA, Davydova MP, Danilov MR, Dyachkova UD, Grigorieva OA, et al. Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. Int J Mol Sci. 2022 Dec 18;23(24):16144.
- Peled E, Sosnik A. Amphiphilic galactomannan nanoparticles trigger the alternative activation of murine macrophages. Journal of Controlled Release. 2021 Nov;339:473–483.
- Verma S, Rimpy, Ahuja M. Carboxymethyl modification of Cassia obtusifolia galactomannan and its evaluation as sustained release carrier. Int J Biol Macromol. 2020 Dec;164:3823–3834.
- Wang Q, Xu W, Koppolu R, van Bochove B, Seppälä J, Hupa L, et al. Injectable thiol-ene hydrogel of galactoglucomannan and cellulose nanocrystals in delivery of therapeutic inorganic ions with embedded bioactive glass nanoparticles. Carbohydr Polym. 2022 Jan;276:118780.
- Alkie TN, Yitbarek A, Taha-Abdelaziz K, Astill J, Sharif S. Characterization of immunogenicity of avian influenza antigens encapsulated in PLGA nanoparticles following mucosal and subcutaneous delivery in chickens. PLoS One. 2018 Nov 1;13(11):e0206324.
- Li Z, Xiong F, He J, Dai X, Wang G. Surface-functionalized, pH-responsive poly(lactic-co-glycolic acid)-based microparticles for intranasal vaccine delivery: Effect of surface modification with chitosan and mannan. European Journal of Pharmaceutics and Biopharmaceutics. 2016 Dec;109:24–34.
- Gonçalves C, Ferreira SA, Correia AL, Lopes C, Fleming CE, Rocha E, et al. Potential of mannan or dextrin nanogels as vaccine carrier/adjuvant systems. J Bioact Compat Polym. 2016 Sep 27;31(5):453–466.
- Weinberger EE, Himly M, Myschik J, Hauser M, Altmann F, Isakovic A, et al. Generation of hypoallergenic neoglycoconjugates for dendritic cell targeted vaccination: A novel tool for specific immunotherapy. Journal of Controlled Release. 2013 Jan;165(2):101–109.
- Khan MUA, Raza MA, Razak SIA, Abdul Kadir MR, Haider A, Shah SA, et al. Novel functional antimicrobial and biocompatible arabinoxylan/guar gum hydrogel for skin wound dressing applications. J Tissue Eng Regen Med. 2020 Oct 17;14(10):1488–1501.
- Yuan Y, Wang L, Mu RJ, Gong J, Wang Y, Li Y, et al. Effects of konjac glucomannan on the structure, properties, and drug release characteristics of agarose hydrogels. Carbohydr Polym. 2018 Jun;190:196–203.
- Li Z, Zeng R, Yang L, Ren X, Maffucci KG, Qu Y. Development and Characterization of PCL Electrospun Membrane-Coated Bletilla striata Polysaccharide-Based Gastroretentive Drug Delivery System. AAPS PharmSciTech. 2020 Feb 13;21(2):66.
- Yuan X, Amarnath Praphakar R, Munusamy MA, Alarfaj AA, Suresh Kumar S, Rajan M. Mucoadhesive guargum hydrogel inter-connected chitosan-g-polycaprolactone micelles for rifampicin delivery. Carbohydr Polym. 2019 Feb;206:1–10.
- Gou M, Dai M, Li X, Yang L, Huang M, Wang Y, et al. Preparation of mannan modified anionic PCL–PEG–PCL nanoparticles at one-step for bFGF antigen delivery to improve humoral immunity. Colloids Surf B Biointerfaces. 2008 Jun;64(1):135–139.
- Wu Q, Gong C, Shi S, Wang Y, Huang M, Yang L, et al. Mannan Loaded Biodegradable and Injectable Thermosensitive PCL-PEG-PCL Hydrogel for Vaccine Delivery. Soft Mater. 2012 Oct;10(4):472–486.
- Laha B, Goswami R, Maiti S, Sen KK. Smart karaya-locust bean gum hydrogel particles for the treatment of hypertension: Optimization by factorial design and pre-clinical evaluation. Carbohydr Polym. 2019 Apr;210:274–288.
- Liu C, Li J, Li K, Xie C, Liu J. Oxidized konjac glucomannan-cassava starch and sucrose esters as novel excipients for sustained-release matrix tablets. Int J Biol Macromol. 2020 Aug;156:1045–1052.
- Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020 Jul 30;39(7):2085–2094.
- Li L, Xu W, Luo Y, Lao C, Tong X, Du J, et al. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr Polym. 2022 Dec;297:120032.
- Kramer S, Langhanki J, Krumb M, Opatz T, Bros M, Zentel R. HPMA‐Based Nanocarriers for Effective Immune System Stimulation. Macromol Biosci. 2019 Jun 10;19(6).
- Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles–mumps–rubella–varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine. 2011 Jun;29(25):4274–4284.
- Chen S, Wang J, Cheng H, Guo W, Yu M, Zhao Q, et al. Targeted Delivery of NK007 to Macrophages to Treat Colitis. J Pharm Sci. 2015 Jul 1;104(7):2276–2284.
- Wen T, Li Y, Wu M, Sun X, Bao X, Lin Y, et al. Therapeutic effects of a novel tylophorine analog, NK‐007, on collagen‐induced arthritis through suppressing tumor necrosis factor α production and Th17 cell differentiation. Arthritis Rheum. 2012 Sep 27;64(9):2896–2906.

 ETFLIN
Notification
ETFLIN
Notification







