Pometia pinnata in Pharmaceutical Research: Bioactivity, Mechanisms, and Formulation Prospects
by Lela Sulastri, Nining Sugihartini ★ , Nuri Ari Efiana
Academic editor: Garnadi Jafar
Sciences of Pharmacy 4(3): 133-150 (2025); https://doi.org/10.58920/sciphar0403331
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
24 Apr 2025
30 May 2025
01 Jul 2025
22 Jul 2025
Abstract: A literature review was conducted to gather information on the pharmacological properties and pharmaceutical dosage forms made from the Matoa plant (Pometia pinnata). The review aimed to provide a basis for further research and explore the potential of Matoa in the health sector. The method involved searching electronic media platforms, specifically Google Scholar, PubMed, and Open Knowledge Maps, using targeted keywords such as "Matoa,” "Pometia pinnata,” "Matoa preparation formulation,” "Pometia pinnata pharmaceutical preparation,” "Preparation of Pometia pinnata," and "Function of Pometia pinnata." The inclusion criteria included original research, full-text articles, and open-access journals, all published within the last 10 years, in both Indonesian and English. After screening, 64 articles were identified from the 185 search results. The review revealed that various parts of the Matoa plant, including leaves, fruit, stem bark, fruit peel, and roots, possess pharmacological properties such as antibacterial, analgesic, antioxidant, sunscreen, anticancer, antidiarrheal, anti-HIV, anti-obesity, diuretic, nephrotoxic, and antihypertensive activities. Additionally, various pharmaceutical dosage forms containing Matoa were studied, including topical preparations such as lotions, creams, liquid soap, and body scrubs, as well as oral preparations like effervescent powders, herbal drinks, and jelly candies. The review suggests that numerous research opportunities remain to further explore the potential of Matoa in the pharmaceutical and healthcare fields, as well as to develop innovative dosage forms for optimal results.
Keywords: Matoa pharmacologyMatoa plant medicinal usesMatoa bioactivityTraditional plant-based medicineEthnopharmacology of matoa
Introduction
Matoa (P. pinnata), a member of the Sapindaceae family, is an endemic plant of Papua and is often regarded as a symbol of Papuan identity. It is widely distributed across the Asia-Pacific region, including Malaysia, Indonesia, the Philippines, Papua New Guinea, the Solomon Islands, Fiji, and Tonga. Within Indonesia, it is found on several major islands, including Java, Sumatra, Kalimantan, and Sulawesi (1). The Matoa plant offers numerous potential benefits in both the health and economic sectors, facilitating its continued development. Papuans have long used the Matoa plant in traditional medicine. The bark is applied to treat burns, festering wounds, and smallpox, while a mixture of the leaves and bark is used to relieve oral infections, flatulence, diarrhea, dysentery, muscle and joint pain, headaches, fever, flu, diabetes, and ulcers (2).
From an economic perspective, the seeds of the Matoa fruit can be utilized as a local food source, and the stem can be utilized in the wood industry (2). The most recognizable and popular part of the Matoa plant is the fruit, which has a delicious taste and aroma (3). Matoa fruit can be marketed through traditional markets and online marketplaces. The availability of Matoa fruit differs from that of seasonal fruit, resulting in fluctuating selling prices (2). Matoa fruit is rich in vitamin C, making it beneficial for skincare. It helps brighten the skin, reduce acne, maintain moisture, and protect against ultraviolet (UV) radiation (2).
The Matoa plant offers numerous benefits, as nearly all of its parts contain secondary metabolites with pharmacological activity. Physicochemical analysis using Liquid Chromatography–Mass Spectrometry (LC-MS) has shown that the ethanol extract of Matoa leaves contains flavonoids such as quercetin and kaempferol, as well as phenolic compounds including gallic acid, phenol, syringic acid, vanillin, p-hydroxybenzaldehyde, and p-coumaroyl glycolic acid (4). In addition, Matoa leaves have been identified to contain alkaloids, tannins, saponins, steroids, and glycosides. The ethanol extract of Matoa stem bark also contains alkaloids, flavonoids, saponins, triterpenoids, and tannins (5). Phytochemical screening has shown that the skin of the Matoa fruit contains alkaloids, flavonoids, phenols, tannins, and saponins. The seeds contain alkaloids, flavonoids, phenols, and tannins, while the flesh of the fruit contains flavonoids, phenols, tannins, and saponins (6).
Despite growing interest in the pharmacological potential of the Matoa plant, current research lacks consistency and standardization across key variables, including extraction methods, solvents, dosages, administration routes, testing procedures, and dosage forms. This variation limits the ability to draw reliable conclusions and hinders the development of evidence-based applications. Furthermore, the exploration of Matoa in pharmaceutical dosage forms remains limited. Therefore, this review aims to synthesize existing findings related to extraction techniques, formulation types, pharmacological testing, and dosage forms of Matoa, providing a comprehensive reference to support more standardized, effective, and targeted future research and development in medicinal applications.
Methodology
Data Collection and Identification
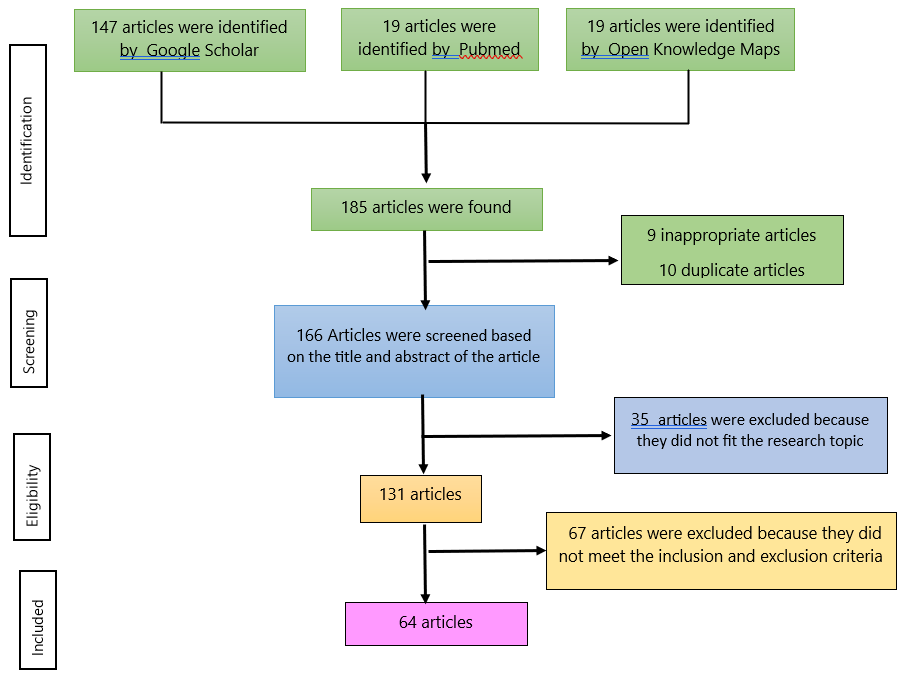
To compile this article, the first step involved searching for relevant literature through electronic databases, including Google Scholar, PubMed, and Open Knowledge Maps. The search was conducted between March and May 2024 using the keywords: "Matoa," "Pometia pinnata," "Matoa preparation formulation," "Pometia pinnata pharmaceutical preparation," "Preparation of Pometia pinnata," and "Function of Pometia pinnata." As a result, 147 articles were identified from Google Scholar, 19 from PubMed, and 19 from Open Knowledge Maps, totaling 185 articles.
Screening Process for Duplicate and Irrelevant Articles
The next stage was the screening process, which involved checking for duplicate entries and excluding articles that were not relevant or did not meet eligibility criteria. This process identified 10 duplicate articles and 9 that were deemed unsuitable, leaving 166 articles to proceed to the selection stage.
Selection Process
The third stage is selection, which involves reading and reviewing titles and abstracts that match the research topic. At this stage, 35 articles were identified that did not match the research topic, and 131 articles had appropriate research topics.
Article Eligibility Assessment
At this stage, an assessment was conducted to determine the suitability of the article's content concerning predetermined inclusion and exclusion criteria. The exclusion criteria include review articles, books, abstracts, closed-access articles, incomplete research extraction methods, activity test methods, and incomplete test parameters.
The inclusion criteria used were original research articles, full-text articles, and open-access articles published between 2014 and 2024, in both Indonesian and English. From 131 articles, 15 closed-access articles, 12 articles that have been reviewed, 9 review articles, 5 books, 9 abstracts, 5 articles with incomplete extraction method data, 7 articles with incomplete test parameter data and 5 articles with incomplete activity test methods were identified. Thus, at this stage 64 eligible articles were obtained for review. The flow of the narrative review process is listed in the prism diagram (see Figure 1).
Data Analysis
Articles that met the inclusion criteria were collected and summarized in a tabular format for analysis of the research objectives and findings. Content analysis was applied to extract key information, followed by coding based on predefined categories. For pharmacological studies, the categories included type of activity, type of extract or fraction, extraction method, activity testing method, dosage, and results. For studies on Matoa dosage forms, the analysis covered the type of formulation, plant part used, extraction method, extract dosage, tested variables, and research outcomes. The collected data were then compared to identify similarities and differences, which were subsequently discussed to draw meaningful conclusions.
No | Extracts & Fractions | Extraction Method | Microorganism(s) | Method | Concentration | Inhibition Zone (mm) | MIC (µg/mL) | MBC/MFC (µg/mL) | NIC (µg/mL) | Inhibition Category | Reference |
1 | Ethanol Extract of Matoa Leaves | Maceration | Shigella sonnei | Disc Diffusion | 100% | 14.67 ± 2.5 | – | – | – | Medium | (7) |
Bacillus cereus | 100% | 13.33 ± 0.5 | – | – | – | Medium | |||||
2 | Ethanol Extract of Matoa Leaves | Maceration | Candida albicans | Disc Diffusion | 70% – 30% | 10.10 – 12.78 | – | – | – | Medium | (8) |
3 | Methanol Extract of Matoa Fruit Flesh | Maceration | E. coli, B. cereus, A. flavus, A. niger | Disc Diffusion | – | – | – | – | – | – | (9) |
Ethyl Acetate Extract of Fruit Pulp | – | – | – | – | Medium/Weak | ||||||
Hexane Extract of Fruit Pulp | – | – | – | – | Medium/Weak | ||||||
4 | Ethanol Extract of Matoa Fruit Bark | Maceration | Staphylococcus aureus | Well Diffusion | 20%, 40%, 60% | 6.6, 8.3, 7.7 | – | – | – | Medium | (10) |
5 | Ethanol Extract of Matoa Leaves | Maceration | Salmonella typhi | Disc Diffusion | 30%, 40%, 50% | 12.6 – 15.3 | – | – | – | – | (7) |
6 | Aqueous Extract of Matoa Fruit Bark | Maceration | E. coli, B. cereus, S. aureus | Disc Diffusion | 0.5–500 ppm | – | – | – | 5 | Weak | (11) |
Acetone Extract of Matoa Fruit Bark | – | – | – | – | <0.5 | Strong | |||||
Ethanol Extract of Matoa Fruit Bark | – | – | – | – | <0.5 | Strong | |||||
7 | Ethanol Extract of Matoa Leaves | Maceration | S. aureus | Disc Diffusion | 1%, 1.5%, 2% | 12.88 – 13.15 | – | – | – | Strong | (12) |
E. coli | 1%, 1.5%, 2% | 9.5 – 31.57 | – | – | – | Medium to Very Strong | |||||
8 | Ethanol Extract of Matoa Fruit Bark | Maceration | Streptococcus mutans | Disc Diffusion | 25%, 50%, 75% | 11.75 – 18.75 | – | – | – | Strong | (13) |
9 | Nanoparticles of Matoa Leaf Ethanol Extract | Maceration | E. coli | Disc Diffusion | 2.5%, 5%, 7.5% | 6.6 – 7.7 | – | – | – | Resistant | (14) |
10 | Ethanol Extract of Matoa Stem Bark | Maceration | S. aureus | Disc Diffusion | 1.5%, 2%, 2.5% | 12.99 – 15.27 | – | – | – | Strong | (15) |
11 | Ethyl Acetate Fraction of Matoa Leaves | Maceration | S. aureus | Disc Diffusion | 10%, 20%, 30% | 8.39 – 12.00 | – | – | – | Medium to Strong | (16) |
12 | Ethanol Extract of Matoa Leaves | Reflux | S. aureus, P. aeruginosa | Disc Diffusion | 12.5–200 µg/mL | 10.16 – 17.31 | – | – | – | – | (17) |
13 | Nanoparticles of Matoa Leaf Ethanol Extract | Maceration | S. mutans | Disc Diffusion | 2.5%, 5%, 7.5% | 8 – 10.1 | – | – | – | Resistant | (14) |
Matoa Plant, Phytochemicals, and Traditional Usage
Antibacterial and Antifungal
Numerous studies have examined the antibacterial and antifungal properties of Matoa using various plant parts, including leaves, fruit pulp, fruit skin, stem bark, and even nanoparticle formulations of Matoa leaf extract, as summarized in Table 1. The most commonly used extraction method across these studies is maceration, which involves soaking the powdered plant material (simplicia) in a suitable solvent at room temperature for several days, protected from light. Common solvents include water, ethanol, methanol, acetone, ethyl acetate, and hexane, each differing in polarity, which influences the types of compounds extracted and the resulting antibacterial activity.
Antibacterial activity was typically assessed using disc diffusion and hole diffusion methods, adapted to the specific properties of the sample. The reviewed studies reported antibacterial effects against a range of bacteria, such as Shigella sonnei, Bacillus cereus, Escherichia coli, Staphylococcus aureus, Salmonella typhi, Streptococcus mutans, Staphylococcus epidermidis, and Pseudomonas aeruginosa, as well as antifungal activity against Candida albicans, Aspergillus flavus, and Aspergillus niger. Notably, ethanol extracts of Matoa leaves obtained via maceration exhibited moderate antibacterial activity against S. sonnei, B. cereus, and C. albicans, as well as strong activity against Salmonella Typhi, S. aureus, and E. coli (7, 13, 19).
Methanol extract of Matoa fruit pulp exhibited no antibacterial activity against E. coli, B. cereus, A. flavus, or A. niger. Similarly, the ethyl acetate extract showed no activity against E. coli but demonstrated moderate antibacterial potential against B. cereus (inhibition zone diameter of 7 mm) and A. flavus (6 mm), as well as weak activity against A. niger (2 mm) (9). In contrast, ethanol and acetone extracts of Matoa fruit peel showed moderate to strong antibacterial activity against S. aureus, E. coli, B. cereus, and S. mutans, while the water extract was much less effective, exhibiting only weak activity against the same organisms (11-13). This contrast in efficacy across solvents and plant parts underscores the importance of both solvent polarity and phytochemical distribution.
Nanoparticle formulations of ethanol extract from Matoa leaves, at concentrations of 2.5%, 5%, and 7.5%, demonstrated antibacterial activity against E. coli and S. mutans; however, they remained in the "resistant" category, with inhibition zone diameters below 11 mm (14). However, the study found that the antibacterial efficacy of a 2.5% nanoparticle extract was comparable to that of a 25% conventional ethanol extract, suggesting that nanoparticle technology can significantly reduce the required dose of the extract. This highlights the critical role of particle size in enhancing solubility, absorption, and cellular penetration. Due to their lower surface tension and energy, nanoparticles are more readily absorbed and can more easily penetrate cell membranes (19).
The antibacterial activity of Matoa is attributed to the presence of secondary metabolites, including flavonoids, tannins, and saponins. Several flavonoids, including apigenin, galangin, flavones, flavonol glycosides, isoflavones, flavanones, and chalcones, have been shown to possess strong antibacterial properties. These compounds typically act through multiple cellular targets rather than a single specific site. One mechanism involves the formation of complexes with proteins via nonspecific interactions, including hydrogen bonding, hydrophobic forces, and covalent bonding (20). Flavonoids can also form complexes with extracellular and soluble proteins, disrupting bacterial cell membranes and leading to the leakage of intracellular contents (7). Their antibacterial mechanisms may also include the inhibition of microbial enzymes, adhesins, and cell envelope transport proteins, as well as disruption of microbial membranes, particularly by lipophilic flavonoids (20).
Saponins exert antibacterial effects by increasing the permeability of bacterial cell membranes, which can lead to cell lysis. This mechanism involves interaction with membrane components, resulting in structural disruption and, ultimately, cell rupture (20). Saponins function as antibacterials by denaturing proteins; their surface properties resemble those of detergents, allowing them to reduce the surface tension of bacterial cell walls and compromise the permeability of bacterial membranes. Saponins permeate the cytoplasmic membrane, disrupting its integrity and leading to cytoplasmic leakage, which ultimately results in cell death (9, 22).
The antifungal potential of Matoa leaf extract against C. albicans has been demonstrated, with ethanol extract showing moderate antifungal activity (8). This effect is attributed to the presence of secondary metabolites such as flavonoids, alkaloids, terpenoids, and saponins, which are known to inhibit fungal growth. Flavonoids, which plants synthesize in response to microbial infection, are effective antifungal agents. Their mechanism involves disrupting fungal cell membrane permeability. The hydroxyl groups present in flavonoids alter organic components and nutrient transport mechanisms, ultimately leading to the lysis of fungal cells (22). Alkaloids exert antifungal effects by inhibiting cellular respiration and interfering with the synthesis of proteins, phospholipid membranes, and nucleic acids. Their antifungal activity is also associated with the disruption of microbial cell walls by interfering with peptidoglycan structure (9, 23). Tannins inhibit fungal growth by preventing the synthesis of ergosterol, the main sterol component of fungal cell membranes. Ergosterol plays a structural and regulatory role in fungal cells, similar to the function of cholesterol in mammalian membranes. By altering membrane permeability, tannins compromise cell integrity and function (24). Saponins act as antifungal agents due to their surfactant properties. These compounds reduce the surface tension of fungal cell membranes, increasing permeability and leading to cell damage or death (25). Research by Ismaini (26) also indicates that triterpenoid compounds present in plant extracts contribute to antifungal activity. These compounds exhibit toxicity to pathogenic fungi by damaging cellular organelles, inhibiting enzymatic activity, and suppressing fungal growth.
Despite the lack of activity in methanol extracts of the pulp, other studies report that pulp extracts can exhibit antimicrobial activity, with the ethyl acetate extract consistently showing the highest potency, followed by hexane and then methanol. This discrepancy may arise from differences in extraction protocols, regional plant chemotypes, or compound degradation during processing. Phytochemical analysis supports the observed trends. The ethyl acetate extract contains a higher concentration of alkaloids, which are known for their antimicrobial effects. Alkaloids, due to their nitrogen-containing structures and basicity, can disrupt microbial viability by inhibiting cell wall synthesis, increasing membrane permeability, and interfering with protein biosynthesis. In fungal cells, alkaloids disrupt peptidoglycan synthesis, impairing structural integrity and leading to cell lysis (9, 27). Additionally, terpenoids, especially triterpenoids and steroids, demonstrate antifungal activity by targeting the cytoplasmic membrane and potentially inhibiting spore formation. Their hydrophobic properties allow them to integrate into and destabilize fungal membranes, leading to cytoplasmic leakage, organelle dysfunction, and impaired proton gradients. These mechanisms explain the observed antifungal effects against A. flavus, A. niger, and B. cereus (28). Thus, while some findings appear contradictory at first glance, they are justified by differences in solvent efficiency, compound solubility, and phytochemical distribution across various parts of the Matoa plant. Future studies should standardize extraction parameters and consider compound profiling to better correlate bioactivity with specific phytoconstituents.
Analgesic
Matoa, especially the leaves and bark, can be used as an alternative pain reliever/analgesic (30-31). Matoa leaves contain secondary metabolites, including flavonoids, tannins, saponins, and steroids (see Table 2). Saponins have an analgesic effect that is thought to inhibit the activity of the enzyme cyclooxygenase 2 (COX-2), which produces prostaglandins as one of the mediators of pain, thereby inhibiting or preventing pain activity. Matoa tree bark contains saponins and tannins. The mechanism of action of tannin as an analgesic involves the inhibition of Cox-2, which in turn inhibits prostaglandin biosynthesis. The analgesic mechanism of action of Matoa bark extract is through cyclooxygenase inhibition, thereby preventing arachidonic acid from converting into cyclic prostaglandin endoperoxide. Cyclic prostaglandin endoperoxide is the precursor of all prostaglandins; therefore, when the compound is not formed, prostaglandin synthesis stops (30).
No | Extract/Fraction | Extraction Method | Assessment Method | Induction | Dose | Result | Reference |
1 | Matoa Leaf Ethanol Extract | Maceration | Number of Wriggles | 1% Acetic Acid (IP) | 200, 400, 800 mg/kg BW | Effective analgesic activity observed at 800 mg/kg BW | (29) |
2 | Matoa Bark Extract | Maceration | Number of Hind Leg Licks or Skips | Heat Shock at 55°C | 50 mg/mouse | Movement response decreased from 22 (baseline) to 19.3 at 30 min, and to 1 by 120 mins | (30) |
No | Extract/Fraction | Extraction Method | Method(s) | Result | Category | Reference |
1 | Matoa Leaf 96% Ethanol Extract | Maceration | DPPH | IC₅₀ = 5.46 ± 0.23 ppm | Very Strong | (31) |
Matoa Leaf Ethyl Acetate Extract | IC₅₀ = 5.77 ± 0.20 ppm | |||||
Matoa Leaf Water Extract | IC₅₀ = 5.62 ± 0.11 ppm | |||||
Ethanol Extract (Matoa + Soursop Leaf 1:1) | IC₅₀ = 9.02 ± 0.22 ppm | |||||
Ethyl Acetate Extract (Matoa + Soursop Leaf 1:1) | IC₅₀ = 4.39 ± 0.04 ppm | |||||
Water Extract (Matoa + Soursop Leaf 1:1) | IC₅₀ = 6.66 ± 0.22 ppm | |||||
2 | N-Hexane Extract of Matoa Leaf | Maceration | DPPH | IC₅₀ = 306.49 µg/mL | Very Weak | (32) |
Matoa Leaf Ethyl Acetate Extract | IC₅₀ = 261.07 µg/mL | |||||
Matoa Leaf 96% Ethanol Extract | IC₅₀ = 1.403 µg/mL | Very Strong | ||||
3 | Matoa Leaf Hexane Fraction | Maceration | DPPH | IC₅₀ = 312.12 µg/mL | Very Weak | (33) |
4 | Methanol Extract of Matoa Bark | Maceration | DPPH | IC₅₀ = 70.93 ppm | Strong | (34) |
5 | Matoa Leaf Methanol Extract | Maceration | DPPH | IC₅₀ = 6.42 ± 0.18 ppm | Very Strong | (35) |
Matoa Leaf Ethanol Extract | IC₅₀ = 8.62 ± 0.66 ppm | Very Strong | ||||
Matoa Leaf Ethyl Acetate Extract | IC₅₀ = 170.64 ± 4.44 ppm | Weak | ||||
6 | Matoa Leaf Ethanol Extract | Maceration | In Vivo (ALT & AST levels post-CCl₄ induction) | ALT/AST decreased with increasing dose (100–300 mg/kg BW), approaching normal values | – | (36) |
7 | Methanol Extract of Matoa Seed Bark | Maceration | DPPH (% inhibition) & ABTS | DPPH = 90.26%, ABTS = 93.91% | Strong | (37) |
Ethyl Acetate Extract of Matoa Seed Skin | DPPH = 90.05%, ABTS = 88.47% | Strong | ||||
N-Hexane Extract of Matoa Seed Shell | DPPH = 7.40%, ABTS = 88.84% | Strong (ABTS), Very Weak (DPPH) | ||||
8 | 95% Ethanol Extract of Matoa Leaf | Reflux | DPPH & ABTS (IC₅₀) | DPPH = 41.83 ± 0.17 µg/mL, ABTS = 151.02 ± 0.21 µg/mL | Very Strong (DPPH), Weak (ABTS) | (17) |
9 | Methanol Extract of Matoa Fruit Bark | Maceration | DPPH (% inhibition) | 30.08% – 47.60% | – | (38) |
Methanol Extract of Matoa Fruit Flesh | 12.12% – 15.73% | – | ||||
Methanol Extract of Matoa Fruit Seed | 27.66% – 35.79% | – | ||||
Ethyl Acetate Extract of Matoa Fruit Bark | 32.38% – 52.08% | – | ||||
Ethyl Acetate Extract of Matoa Fruit Pulp | 29.27% – 32.23% | – | ||||
Ethyl Acetate Extract of Matoa Fruit Seed | 27.18% – 30.44% | – | ||||
N-Hexane Extract of Matoa Fruit Bark | 26.20% – 41.76% | – | ||||
N-Hexane Extract of Matoa Fruit Flesh | 22.88% – 35.70% | – | ||||
N-Hexane Extract of Matoa Fruit Seed | 28.64% – 36.46% | – | ||||
10 | Ethanol Extract of Matoa Leaf | Maceration | Liver SOD activity (post-CCl₄ exposure) | SOD levels: 1253.79 ± 5.07, 1411.11 ± 64.68, 1376.57 ± 12.06 U/mg | Increased liver SOD | (39) |
11 | Acetone, Ethanol, Water Extracts of Matoa Fruit Bark | Maceration | DPPH | Acetone IC₅₀ = 15.32 ppm (Very Strong), Ethanol = 143.13 ppm (Weak), Water = 451.31 ppm (Very Weak) | Mixed | (11) |
12 | N-Hexane, Ethyl Acetate, Ethanol Extracts (Leaf) | Maceration | DPPH (% inhibition at 100 ppm) | N-Hexane = 60.81%, Ethyl Acetate = 69.55%, Ethanol = 90.38% | Strong | (40) |
13 | Matoa Leaf 96% Ethanol Extract | Maceration | DPPH | IC₅₀ = 45.78 µg/mL | Strong | (41) |
14 | Ethanol Extract of Matoa Fruit Bark | Modified QuEChERS | DPPH & TBARS (MDA levels) | IC₅₀ = 6.6 µg/mL, Lipid peroxidation = 1.9 ± 0.8 nmol MDA/mg linoleic acid | Very Strong | (42) |
15 | Ethanol 96% Extract of Matoa Fruit | Maceration | DPPH | IC₅₀ = 181.55 ppm | Weak | (43) |
Antioxidants
Based on the review findings (see Table 3), numerous studies have investigated the antioxidant activity of various parts of the Matoa plant, including the leaves, stem bark, seed coat, fruit skin, fruit flesh, and seeds. Antioxidant activity is typically expressed as a percentage of inhibition or by IC₅₀ values, where a lower IC₅₀ indicates stronger antioxidant potential. Antioxidant strength is classified as very strong for IC₅₀ < 50 ppm, strong for 50–100 ppm, moderate for 100–150 ppm, and weak for 150–200 ppm (44).
Several studies report that Matoa leaves exhibit high antioxidant activity when extracted using the maceration method. One study using ethanol distillate yielded an IC₅₀ of 45.78 µg/mL, categorized as very strong (41). Other research by Baslani et al., Islami et al., and Wulandari et al., using 96% ethanol via maceration, reported IC₅₀ values of 5.46 ± 0.23 ppm, 8.622 ± 0.66 ppm, and 1.403 µg/mL, respectively, all classified as very strong antioxidants. Additionally, a study using 95% ethanol obtained an IC₅₀ of 41.83 ± 0.17 µg/mL and an inhibition rate of 90.38%, further confirming the strong antioxidant potential of Matoa leaves (14, 32, 33, 36).
Matoa leaves extracted via maceration using ethyl acetate and its ethyl acetate fraction exhibit very strong antioxidant activity, with IC₅₀ values of 5.77 ± 0.20 ppm and 12.19 µg/mL, respectively (31, 33). The water extract of Matoa leaves also exhibits very strong activity, with an IC₅₀ of 5.62 ± 0.11 ppm (31). In contrast, the n-hexane extract and fraction exhibit significantly weaker activity, with IC₅₀ values of 306.49 µg/mL and 312.12 µg/mL, respectively. In comparison, the chloroform fraction shows moderate activity with an IC₅₀ of 100.95 µg/mL (32, 33). The methanol extract of Matoa stem bark demonstrates strong antioxidant activity, with an IC₅₀ of 70.93 ppm (34). Matoa seed skin extracted using methanol, ethyl acetate, and n-hexane showed DPPH inhibition percentages of 90.26%, 90.05%, and 7.40%, respectively, indicating that the methanol extract had the highest antioxidant activity (37). Research on fruit peels extracted with acetone, ethanol, and water showed IC₅₀ values of 15.32 ppm (very strong), 143.13 ppm (weak), and 451.31 ppm (very weak), respectively (11). An additional study using the QuEChERS method reported a very strong antioxidant activity with an IC₅₀ of 6.6 µg/mL (42). Matoa fruit pulp and seeds extracted via maceration with methanol, ethyl acetate, and n-hexane had weak to very weak activity: IC₅₀ values for the pulp were 12790 µg/mL, 7170 µg/mL, and 2160 µg/mL, and for the seeds, 2720 µg/mL, 2688 µg/mL, and 7656 µg/mL, respectively (38, 43). Based on these findings, the most effective antioxidant source from P. pinnata appears to be its leaves, particularly when extracted via maceration using 96% or 70% ethanol, both of which yield very strong antioxidant activity.
The potent antioxidant properties of Matoa are attributed to its secondary metabolites, particularly phenolic compounds such as flavonoids and tannins, which have been identified through phytochemical screening. Phenolic antioxidants, particularly flavonoids, play a critical role in mitigating oxidative stress by scavenging excess free radicals, thus preventing cellular damage (45). The antioxidant mechanism of flavonoids involves donating hydrogen atoms to peroxyl radicals (ROO•), thereby terminating lipid peroxidation. The resulting phenoxyl radical (PP•) can be stabilized through further hydrogen donation, forming quinone-like structures, or by dimerizing with other radicals, thereby reducing further radical propagation (46).
Sunscreen
Based on the reviewed research (see Table 4), Matoa leaves extracted with ethanol using the Mansur method produced SPF values ranging from 8 to 29, indicating maximum to ultra protection sunscreen activity (47, 48). The sunscreen efficacy of ethanol extracts from Matoa leaves is attributed to the presence of flavonoids, as identified in phytochemical screening (48). These secondary metabolites, particularly phenolic compounds, exhibit photoprotective properties due to their chromophore groups, which include conjugated double and single bonds, allowing them to absorb ultraviolet (UV) radiation, including both UVA and UVB. By absorbing UV rays, flavonoids reduce the penetration of harmful radiation into the skin, thereby preventing UV-induced damage (49).
Tyrosinase Inhibitory Effect
Tyrosinase inhibitors work by blocking the activity of the tyrosinase enzyme, a key catalyst in the melanogenesis process, which leads to melanin production in the basal layer of the stratum corneum. These inhibitors are commonly used to treat hyperpigmentation disorders, where the skin appears darker due to overproduction or uneven distribution of melanin. One of the main triggers of hyperpigmentation is excessive sun exposure, which stimulates melanocyte activity and accelerates melanin synthesis (50).
Matoa is a natural source with potential as an alternative tyrosinase inhibitor. This activity is attributed to the presence of bioactive compounds such as phenols, flavonoids, and glabridin, which have demonstrated inhibitory effects on tyrosinase. Flavonoids act as antioxidants by neutralizing reactive oxygen species (ROS), thereby interrupting the ROS-induced melanogenesis pathway (52).
Additionally, phenolic compounds, including flavonoids, can inhibit tyrosinase through competitive inhibition by mimicking the enzyme's natural substrate and binding to its active site (53). When flavonoids occupy the active site, they block the enzyme from catalyzing the formation of dopachrome, a key intermediate in melanin synthesis. A high level of dopachrome indicates low inhibitory activity, whereas reduced dopachrome formation reflects effective tyrosinase inhibition. The effectiveness of tyrosinase inhibitors is measured by the IC₅₀ value, which represents the concentration required to inhibit 50% of the enzyme's activity. A lower IC₅₀ value indicates stronger inhibitory potential and greater efficacy in suppressing melanin formation (54).
Antihyperuricemia
Based on the review findings (see Table 5), the effective dose of Matoa leaf ethanol extract for reducing uric acid levels is 200 mg/kg body weight (55). The ethanol extract is believed to contain flavonoid compounds that function as antihyperuricemic agents by inhibiting the activity of the enzyme xanthine oxidase, which plays a key role in the conversion of purine bases into uric acid (56).
Hyperuricemia is a condition characterized by elevated levels of uric acid in the blood. Uric acid is a byproduct of purine metabolism, and its accumulation is often associated with metabolic disorders. Research indicates that approximately 90% of uric acid is generated through purine catabolism, primarily involving the enzymes guanase and xanthine oxidase (57).
Antidiabetics
According to the findings of the review (see Table 6), Matoa exhibits antidiabetic activity through multiple mechanisms, including the inhibition of α-glucosidase by extracts of the fruit flesh and methanol, ethyl acetate, and hexane extracts of Matoa seeds, as well as α-amylase inhibition by Matoa seed and seed coat extracts (10, 59, 60). The chloroform fraction of Matoa fruit seeds, administered at 300 mg/kg BW, effectively lowered blood glucose levels in mice. In addition, the 96% ethanol extract of Matoa leaves significantly reduced glucose levels in streptozotocin-nicotinamide-induced diabetic rats, with the most effective dose being 392 mg/kg BW. This dose not only decreased blood glucose but also lowered creatinine levels and improved kidney function in diabetic nephropathy models (57-58).
No | Extract/Fraction | Extraction Method | Method | Result (SPF) | Category | Reference |
1 | Ethanol Extract of Matoa Leaf | Maceration | Mansyur Method | 8.15 | Maximum Protection | (47) |
18.19 | Ultra Protection | |||||
27.97 | Ultra Protection | |||||
29.27 | Ultra Protection | |||||
2 | 50% Ethanol Extract of Matoa Leaf | Maceration | Mansyur Method | 14.09 ± 0.1 | Maximum Protection | (48) |
70% Ethanol Extract of Matoa Leaf | 24.45 ± 0.2 | Ultra Protection | ||||
96% Ethanol Extract of Matoa Leaf | 18.99 ± 0.2 | Ultra Protection |
No | Extract/Fraction | Extraction Method | Method | Induction Protocol | Dose | Day-0 Uric Acid Level (mg/dL) | Post-Induction Level (mg/dL) | Post-Treatment Level (mg/dL) | AUC Mean | Reference |
1 | Matoa Leaf Ethanol Extract | Maceration | Measurement of uric acid levels in male white rats across 5 groups | 7 days of chicken liver juice, followed on day 8 by 250 mg/kg BW intraperitoneal potassium oxonate (IP) | 200 mg/kg BW | 3.60 ± 0.45 | 7.93 ± 2.32 | 6.00 ± 2.28 | 6.96 | (55) |
400 mg/kg BW | 3.80 ± 0.10 | 8.46 ± 1.36 | 6.03 ± 3.25 | 7.25 | ||||||
800 mg/kg BW | 4.10 ± 0.17 | 9.10 ± 9.00 | 14.13 ± 5.75 | 11.61 |
No | Extract/Fraction | Extraction Method | Method(s) | Dose | Induction | Result | Description | Reference |
1 | Methanol Extract of Matoa Fruit Flesh | Maceration | α-Glucosidase Inhibition (IC₅₀) | – | – | IC₅₀ = 341.93 ± 5.02 µg/mL | Ethyl acetate extract showed the highest α-glucosidase inhibition | (9) |
Ethyl Acetate Extract of Matoa Fruit Pulp | – | – | IC₅₀ = 159.74 ± 0.65 µg/mL | |||||
Hexane Extract of Matoa Fruit Pulp | – | – | IC₅₀ = 207.32 ± 1.97 µg/mL | |||||
2 | Hexane Extract of Matoa Fruit Seeds | Multiple Maceration | Sucrose Tolerance Test, Blood Glucose Measurement (glucometer) | 100, 200, 300 mg/kg BW | 90% Sucrose | Glucose level: 186.67, 165.66, 134.67 mg/dL (dose-dependent reduction) | 300 mg/kg BW of chloroform extract showed significant antidiabetic effect | (58) |
Chloroform Extract of Matoa Fruit Seeds | – | – | – | |||||
Ethyl Acetate Extract of Matoa Fruit Seeds | – | – | – | |||||
3 | Ethanol 96% Extract of Matoa Leaf | Maceration | Blood glucose, creatinine levels, histopathology of kidneys in rats | 98, 196, 392 mg/kg BW | Streptozotocin–Nicotinamide | Glucose levels: 153 ± 1.6, 138 ± 1.0, 125.49 ± 1.8 mg/dL. Creatinine lowered by 392 mg/kg BW | Effective at 392 mg/kg BW; lowered blood glucose, creatinine, and improved kidney histology in diabetic nephropathy rats | (59) |
Ethanol 50% Extract of Matoa Leaf | GOD-PAP glucose test, ELISA insulin test, HE pancreas histopathology | 50, 100, 200 mg/kg BW | Alloxan | FBG = 135.25 ± 21.14 mg/dL at 200 mg/kg BW. Insulin ↑ by 0.14%. Fewer damaged β-cells. | 14-day administration at 200 mg/kg BW improved blood glucose, insulin, and β-cell condition in diabetic rats | (60) | ||
4 | Methanol Extract of Matoa Seed | Maceration | α-Amylase Inhibition (Buthkar Method, LC₅₀) | – | – | LC₅₀ = 12.92 ppm | Matoa seed and skin extracts showed α-amylase inhibition | (61) |
Ethyl Acetate Extract of Matoa Seed | – | – | LC₅₀ = 446.43 ppm | |||||
Methanol Extract of Matoa Seed Skin | – | – | LC₅₀ = 4.92 ppm | |||||
Ethyl Acetate Extract of Matoa Seed Skin | – | – | LC₅₀ = 197.24 ppm | |||||
5 | Methanol Extract of Matoa Fruit Seeds | Maceration | α-Glucosidase Inhibition Assay | – | – | IC₅₀ = 169.81 µg/mL | Pometia pinnata seed extract shows promise as an antidiabetic biomedicine | (62) |
Ethyl Acetate Extract of Matoa Fruit Seeds | – | – | IC₅₀ = 505.55 µg/mL | |||||
N-Hexane Extract of Matoa Fruit Seeds | – | – | IC₅₀ = 263.18 µg/mL |
The antidiabetic effects of Matoa fruit and seeds are attributed to the presence of secondary metabolites, particularly flavonoids and alkaloids. Flavonoids have been widely reported to exert antidiabetic effects through several mechanisms. One primary mechanism is the inhibition of the intestinal α-glucosidase enzyme, which slows glucose absorption. The inhibitory activity is closely related to the flavonoid structure; for example, the hydroxyl group at the C3 position plays a crucial role. The greater the number of hydroxyl groups, the stronger the inhibition of α-glucosidase (63).
Flavonoids also inhibit GLUT2 transporters in the intestinal mucosa, reducing the absorption of glucose and fructose into the bloodstream. Additionally, flavonoids inhibit phosphodiesterase, leading to increased intracellular cAMP in pancreatic β-cells, which activates protein kinase A (PKA) and subsequently enhances insulin secretion. Moreover, flavonoids can improve insulin sensitivity and inhibit the α-amylase enzyme, further contributing to reduced postprandial blood glucose levels (58).
Alkaloids, another key bioactive group in Matoa, have been shown to regenerate damaged pancreatic β-cells and stimulate insulin secretion through sympathomimetic effects. Their antidiabetic mechanisms include increasing glucose transport, inhibiting glucose absorption in the intestine, stimulating glycogen synthesis, and suppressing gluconeogenesis by inhibiting enzymes such as glucose-6-phosphatase and fructose-1,6-bisphosphatase. Inhibition of these enzymes reduces glucose production from non-carbohydrate sources (64). Additionally, alkaloids inhibit α-glucosidase in the duodenal mucosa, delaying the breakdown of polysaccharides into monosaccharides. This results in slower glucose release and absorption, preventing sharp postprandial spikes in blood glucose levels (65).
Anti-inflammatory
Research has shown that Matoa leaves, particularly in the form of ethanol extract obtained through maceration, possess anti-inflammatory activity. In animal studies, doses of 100 mg/kg BW, 200 mg/kg BW, and 300 mg/kg BW were tested, with the most significant inhibition of paw edema in mice observed at 300 mg/kg BW, indicating this as the most effective dose among those tested (66).
The anti-inflammatory potential of Matoa leaves is attributed to their rich content of bioactive secondary metabolites, including flavonoids, tannins, saponins, steroids, and glycosides, as identified through phytochemical screening (67). These compounds, particularly flavonoids and tannins, are known for their antioxidant and anti-inflammatory properties.
While the precise mechanism of the anti-inflammatory action of saponins remains not fully understood, it is believed that their steroid or triterpenoid aglycone structures, which exhibit detergent-like properties, enable them to interact with lipid membranes. These interactions may affect phospholipid components, which are precursors of prostaglandins and other inflammatory mediators, thereby contributing to an anti-inflammatory effect (68).
Antihypertension
From Table 7, it can be seen that Matoa leaves in the form of extracts and fractions have activity as antihypertensives with parameters of lowering blood pressure, reducing blood flow circulation (blood flow), and the ability to inhibit the activity of the enzyme γ amylase (IC 50) to determine angiotensin I levels by ELISA method (67, 68, 70). Its efficacy as an antihypertensive is due to Matoa containing flavonol groups, namely Quercetin-3-O-rhamnoside and Kaemferol 3-O-rhamnoside (70).
Research on Quercetin-3-Orhamnoside and Kaemferol 3-O-rhamnoside compounds has also been conducted by Marunaka et al in 2017, and suggested that the Quercetin-3-O-rhamnoside and Kaemferol 3-O-rhamnoside compounds have the potential to reduce blood pressure, with a high vasodilator effect and reduce the severity of hypertension in organs such as the kidneys and arterial and venous blood vessels, using animal and human subjects. The mechanismof lowering blood pressure involves reducing oxidative stress and the renin-angiotensin-aldosterone system (RAAS) (74).
No | Extract/Fraction | Extraction Method | Method(s) | Induction | Dose(s) | Result | Description | Reference |
1 | N-Hexane Fraction | Reflux | Blood pressure (systolic/diastolic) measured using noninvasive tail-cuff (CODA) | NaCl 50 mg/kg BW + Prednisone 1.5 mg/kg BW orally for 28 days | 50, 100, 150 mg/kg BW | MLE effective dose = 150 mg/kg BW. Ethyl acetate fraction effective dose = 13.06 mg/kg BW. | Extracts and fractions of P. pinnata leaves reduced BP in NaCl-prednisone-induced hypertensive rats | (69) |
Ethyl Acetate Fraction | 4.35, 8.71, 13.06 mg/kg BW | |||||||
Water Fraction | 10, 21.88, 32.82 mg/kg BW | |||||||
2 | Matoa Leaf Ethanol Extract | Maceration | % Reduction in blood pressure | Angiotensin II | 75, 150, 300 mg/kg BW | 44.47%, 53.36%, 67.35% reduction (respectively) | 300 mg/kg BW dose showed highest reduction in circulating blood flow | (70) |
3 | Matoa Leaf Ethanol Extract | Maceration | Blood volume via tail-cuff method using CODA instrument | Angiotensin II | 75, 150, 300 mg/kg BW | AUC: 10.04 ± 10.57, 14.15 ± 4.10, 31.91 ± 8.40 | Effective dose to reduce blood volume: 300 mg/kg BW | (71) |
4 | N-Hexane, Ethyl Acetate, Water Fractions | Reflux | Angiotensin I levels by ELISA (Cusabio); IC₅₀ inhibition of γ-amylase activity | Angiotensin II | EDM: 60 mg/200 g BW. N-Hexane: 2.34 mg/g. N-Hexane: 2.34 mg/g. Ethyl Acetate: 9.54 mg/200 g. Water: 7.98 mg/200 g. | Angiotensin I inhibition: EDM = 17.2%, Ethyl Acetate = 23.6%, Water ≈ Positive Control | Water fraction (7.98 mg/200 g BW) matched angiotensin I reduction by Irbesartan (positive control) | (72) |
5 | Matoa Leaf Ethanol Extract, N-Hexane Fraction, Ethyl Acetate Fraction, Water Fraction | Maceration (96% ethanol) | Systolic/diastolic BP via CODA; SOD and GPx levels in liver | Angiotensin II | 300 mg/kg BW | Reduced BP, Increased SOD and GPx levels in liver | Extracts and fractions reduced BP and improved antioxidant enzyme levels in angiotensin II-induced Wistar rats | (73) |
Secondary metabolites Quercetin-3-O-rhamnoside and Kaemferol 3-O-rhamnoside are known to be effective as antihypertensives by acting as vasodilators in arteries, which also have the potential to reduce circulating blood flow through a mechanism of lowering blood pressure. The potential of this compound is reinforced by research indicating that Matoa leaves contain Quercetin-3-O-Rhamnoside and Kaemferol 3-O-rhamnoside compounds, which are effective in reducing circulating blood flow through a mechanism of lowering blood pressure. This compound has been clinically tested, and a significant dose for lowering blood pressure is 150 mg-730 mg per day, administered orally for 4 to 5 weeks (75).
Diuretic Effect
In Table 8, it can be seen that Matoa has a diuretic effect, specifically increasing urine output and the excretion of sodium salts. This Study Examined the parts used from Matoa, namely leaves, skin, and Matoa seeds, and showed antidiuretic activity in male Wistar rats. Matoa leaf, skin, and seed extracts can affect the amount of sodium and potassium levels in urine excretion. The effective dose of ethanol extract of Matoa leaves for diuretic effect is 100 mg/kg BW (69;76). The test parameters were the decrease in urine volume and its effect on potassium and sodium levels in urine. The results showed that Matoa seed ethanol extract at 150 mg/kg bw had the highest diuretic activity, but the effect on excretion of sodium and potassium ions was lower (69). In general, Matoa seed extract has diuretic activity but is not saluretic, while Matoa leaf extract has diuretic and saluretic effects at a dose of 100 mg/kg bw. Increasing the amount of potassium in the blood results in reduced renin secretion and increased Na+ excretion. Matoa plants contain alkaloid and flavonoid compounds, which inhibit or reduce the reabsorption of water and electrolytes in the tubules, causing a diuretic effect (77). The mechanism of action of flavonoids as a diuretic involves inhibiting co-transport and reducing the reabsorption of Na+, K+, and Cl- ions, resulting in an increase in electrolytes in the tubules, which facilitates diuresis (78).
No | Extract(s) | Extraction Method | Methods | Induction | Dose(s) | Result | Description | Reference |
1 | Matoa Leaf Ethanol ExtractMatoa Bark ExtractMatoa Fruit Seed Extract | Maceration | - Measurement of urine volume over 4 h- Determination of sodium and potassium levels in urine using atomic absorption spectrophotometry | Warm water 4 mL/200 g BW | 50 mg/kg BW100 mg/kg BW150 mg/kg BW | All extracts showed a diuretic effect. The 100 mg/kg BW dose produced the highest urine volume.Sodium and potassium levels in all treated groups (except MSE 50 mg/kg) were not significantly different from furosemide. | Matoa leaf, bark, and seed extracts can increase urinary sodium and potassium excretion. The most effective dose for diuretic activity is 100 mg/kg BW. | (69) |
2 | Matoa Leaf 70% Ethanol Extract | Maceration | - Measurement of urine volume at 1, 2, 3, 4, 5, and 24 h- Diuretic effect calculated as % of urine volume at each time point | Water | 50 mg/kg BW100 mg/kg BW150 mg/kg BW | The 100 mg/kg BW dose produced a urine volume and diuretic percentage comparable to the positive control (furosemide 3.6 mg/kg BW). | Matoa leaf extract at 100 mg/kg BW is the most effective dose, showing diuretic activity similar to that of standard drug furosemide. | (76) |
No | Extract | Extraction Method | Methods | Induction | Dose(s) | Lab Results | Description | Reference |
1 | Ethanol Extract of Matoa Fruit | Maceration | Biochemical parameters (urea, creatinine, uric acid, sodium, potassium, chloride, NGAL) and oxidative stress markers (SOD, MDA) via ELISA | Cisplatin 7 mg/kg BW | 100 mg/kg BW | Urea: 54.58 ± 3.81 mg/dL; Creatinine: 1.47 ± 0.29 mg/dL; Uric Acid: 1.58 ± 0.18 mg/dL; Sodium: 205.66 ± 19.86 mEq/L; Potassium: 18.48 ± 1.86 mEq/L; Chloride: 250.56 ± 12.65 mEq/L; NGAL: 0.4237 ± 0.151 ng/mL; SOD: 15.67 ± 0.86 U/mL; MDA: 10.44 ± 0.23 nmol/mL | Moderate nephroprotective effect; partial improvement in renal function and oxidative stress markers. Clear dose-dependent nephroprotective effect; 400 mg/kg BW showed the greatest normalization of renal biomarkers and antioxidant activity. | (79) |
200 mg/kg BW | Urea: 48.42 ± 3.16 mg/dL; Creatinine: 0.91 ± 0.073 mg/dL; Uric Acid: 1.02 ± 0.098 mg/dL; Sodium: 152.88 ± 12.41 mEq/L; Potassium: 10.42 ± 0.84 mEq/L; Chloride: 185.18 ± 10.44 mEq/L; NGAL: 0.347 ± 0.019 ng/mL; SOD: 16.58 ± 0.93 U/mL; MDA: 6.45 ± 0.081 nmol/mL | |||||||
400 mg/kg BW | Urea: 32.41 ± 2.62 mg/dL; Creatinine: 0.78 ± 0.062 mg/dL; Uric Acid: 0.65 ± 0.044 mg/dL; Sodium: 140.40 ± 10.51 mEq/L; Potassium: 5.28 ± 0.46 mEq/L; Chloride: 95.86 ± 8.03 mEq/L; NGAL: 0.1217 ± 0.021 ng/mL; SOD: 24.62 ± 1.05 U/mL; MDA: 5.47 ± 0.058 nmol/mL |
Nephroprotective Activity
Nephrotoxicity is often marked by acute renal failure, typically indicated by elevated serum creatinine and urea levels. As the clinical use of cisplatin, a potent chemotherapeutic agent, continues to grow, efforts to mitigate its nephrotoxic side effects have become increasingly important. One of the main strategies involves reducing elevated levels of serum creatinine and urea to preserve kidney function.
A study demonstrates that Matoa fruit exhibits nephroprotective activity in rats treated with cisplatin (see Table 9), showing a significant reduction in biochemical markers such as urea, creatinine, uric acid, sodium, potassium, chloride, NGAL (Neutrophil Gelatinase-Associated Lipocalin), and MDA (Malondialdehyde), along with an increase in SOD (Superoxide Dismutase) levels (79).
Cisplatin-induced nephrotoxicity results from a combination of inhibited protein synthesis, DNA damage, mitochondrial dysfunction, and apoptosis in renal tubular cells. It also reduces the activity of protective factors such as nitric oxide, monocyte chemoattractant protein-1, and various growth factors, while increasing tumor necrosis factor-α (TNF-α) and reactive oxygen species (ROS), ultimately leading to inflammation and renal injury (80).
The most commonly used biomarkers to evaluate kidney damage include urea and creatinine, as their elevated levels often indicate both structural and functional impairment of the kidneys. The nephroprotective effects of Matoa fruit are thought to be due to its rich content of antioxidant secondary metabolites, particularly flavonoids, phenolics, and alkaloids. These compounds work by neutralizing free radicals and reducing oxidative stress, thereby helping to lower serum creatinine and urea levels and protect kidney tissue from further damage (81).
Antidiarrhea
Matoa ethanol leaf extract, obtained through maceration, has been shown to reduce the frequency and duration of diarrhea. Studies reported that ethanol extracts of Matoa leaves administered at doses of 200, 300, 400, and 800 mg/kg BW significantly decreased diarrhea symptoms, with the 800 mg/kg BW dose demonstrating the most pronounced antidiarrheal effect (82, 83).
The antidiarrheal activity of Matoa leaves is primarily attributed to their tannin content, which acts by astringently shrinking the intestinal mucosa, thereby reducing the intestinal secretion of fluids and electrolytes. Tannins also help make the intestinal lining more resistant to chemical irritants. Additionally, flavonoids present in the extract contribute to this effect by inhibiting intestinal motility, which further helps reduce fluid loss (82).
Studies on Matoa reveals that nearly all parts of the plant, including the leaves, fruit, stem bark, fruit peel, and roots, possess medicinal properties. These effects are supported by phytochemical screenings, which consistently identify various bioactive secondary metabolites in Matoa extracts. Specifically, ethanol extracts of the stem bark and leaves contain compounds such as alkaloids, flavonoids, glycosides, saponins, tannins, terpenoids, and phenolic compounds, including steroids (32, 84).
LC-MS (Liquid Chromatography-Mass Spectrometry) analysis of ethanol extracts of Matoa leaves has identified 12 active compounds, including several phenolic compounds such as vanillin, p-hydroxybenzaldehyde, p-coumaroyl glycolic acid, syringic acid, gallic acid, phenol, and vanillic acid. The flavonoid compounds detected include epigallocatechin and apigenin-7-O-diglucuronide. Other identified components include jasmonic acid (an organic acid), benzene (an aromatic compound), and tannins. Among these, vanillin, phenol, and benzene were the most dominant (4).
Anticancer
The ethanol extract of Matoa leaves, obtained through maceration with 96% ethanol, contains several bioactive secondary metabolites, including alkaloids, flavonoids, tannins, saponins, glycosides, and steroids, which contribute to its potential anticancer activity. Anticancer evaluation of the extract was conducted both in silico and in vitro.
In silico analysis involved physicochemical, pharmacokinetic, and toxicity predictions using Lipinski’s Rule of Five, pkCSM, and ProTox-II. Molecular docking studies were performed with AutoDock Vina and DOCK6.2, followed by molecular dynamics simulations using GROMACS. These analyses demonstrated that 5 out of 20 identified compounds exhibited strong and stable interactions with the CHK1 (Checkpoint Kinase 1) protein. These interactions included hydrogen bonding and hydrophobic contacts with key amino acid residues, as indicated by favourable binding affinities, grid scores, and MMPBSA energy values.
To validate these findings, in vitro cytotoxicity testing was carried out against MCF-7 breast cancer cells using the MTT assay. The ethanol extract exhibited dose-dependent cytotoxic effects, with increasing concentrations resulting in a marked reduction in cell viability. The extract demonstrated a moderate IC₅₀ value, indicating potential anticancer activity against MCF-7 cells (85).
Antiobesity
Matoa fruit peel is rich in phenolic and polyphenolic compounds, which contribute to its antioxidant properties and potential to combat obesity. In one study, Matoa fruit bark was extracted using ethanol extraction, followed by sonication, filtration, concentration, and vacuum drying, yielding a greenish-yellow crude extract.
When Matoa fruit peel powder was incorporated at 1% (w/w) into a high-fat diet (HFD) fed to Sprague-Dawley rats, it showed no significant effects on body weight, visceral fat weight, or serum glucose and lipid levels. However, at this dose, it significantly reduced hepatic lipid accumulation. Increasing the dosage to 3% (w/w) resulted in a significant reduction in body weight, visceral fat, serum triglyceride levels, and liver lipid content, demonstrating a dose-dependent anti-obesity effect.
Interestingly, increasing the concentration of the Matoa peel extract from 1% to 3% did not enhance its inhibitory effect on hepatic lipid accumulation, suggesting a threshold effect. The anti-obesity mechanism may be partially attributed to the inhibition of fatty acid-induced ApoB-48 secretion, a marker of intestinal chylomicron formation, observed in differentiated Caco-2 cell monolayers. Additionally, hederagenin-type saponins, identified in Matoa fruit bark, were suggested as active anti-obesity compounds.
These findings support the potential of Matoa fruit peel extract as a functional food ingredient with anti-obesity properties (86).
Anti HIV
The leaf extract of Matoa exhibited the strongest anti-HIV-1 integrase (IN) activity, with an IC₅₀ value of 8.8 mg/mL. Bioassay-guided isolation of active compounds from the leaf extract resulted in the identification of a single major active compound, proanthocyanidin A2, which demonstrated notable anti-HIV-1 activity with an IC₅₀ of 30.1 µM.
In addition to proanthocyanidin A2, several other compounds were isolated, including three flavonoids: epicatechin, kaempferol-3-O-rhamnoside, and quercetin-3-O-rhamnoside; one aglycolipid: 1-O-palmitoyl-3-O-[α-D-galactopyranosyl-(1→6)-β-D-galactopyranosyl]-sn-glycerol; one steroidal glycoside: stigmasterol-3-O-glucoside; and one pentacyclic triterpenoid saponin: 3-O-α-L-arabinofuranosyl-(1→3)-[α-L-rhamnopyranosyl-(1→2)]-α-L-arabinopyranosyl hederagenin.
No | Type of preparation | Parts used | Extraction method | Extract Concentration | Variable | Result | Reference |
1 | Lotion | Matoa fruit bark extract | 96% ethanol maceration | 1.5% and 2% | Independent variable: 1.5% and 2% extract concentration Dependent variable: Stability of lotion | Lotions 1.5% and 2% are stable in organoleptic test parameters, homogeneity, spreadability and flow properties and unstable in pH and viscosity parameters. | (87) |
2 | Masker Peel Off | Peel Off Mask | Ethanol 96% | 2% | Independent variable: gelling agent PVA 5%, 8%, 10%, 12% and 15% Dependent variable: Physical Characteristics | Variations in PVA concentration cause differences in preparation characteristics. Brownish green color, distinctive odor, bitter taste, homogeneous, viscosity 50-110 dPa, pH according to skin pH 4.5-6.5, spreadability in the range of 5-7 cm with an area of 13-58 cm2, adhesion 17-36 s. | (88) |
3 | Gel | Matoa Leaf Ethanol Extract | 96% ethanol maceration | 2,3,and 4 % | Independent variable: 2, 3, and 4% extract concentration Dependent variable: physical characteristics | The physical evaluation results meet the requirements: homogeneity, pH, spreadability, organoleptic. | (89) |
N Hexan and Ethanol Extracts of Matoa Leaf | 96% Ethanol and N hexane maceration | 2.5, 5 and 10% | Independent variable: 2.5, 5 and 10% extract concentration Dependent variable : Tricophyton mentagrophy tes antifungal activity tes | N-hexan and ethanol extract gels have inhibitory power as antifungall and formula 3 (10%) is the best in inhibiting the growth of Trichophyton fungus with an average inhibition zone diameter of 18.22 mm. | (90) | ||
4 | Body Scrub | Matoa Leaf Ethanol Extract | 96% ethanol maceration | 1%, 2.5%, 4% and 5.5% | Independent variables: extract concentrations of 1,2,5 4 and 5.5% Dependent variables: physical characteristics of the preparation, antioxidant activity (IC50) and Irritation Index | Concentration 5.5 IC 50 44.49 ppm (very strong) and all formulas meet the physical evaluation requirements (organoleptic, pH, spreadability, homogenization) and are non-irritating. | (91) |
5 | Krim | Matoa Leaf Ethanol Extract | 96% ethanol maceration | 0.5%, 1 %, 1.5% dan 2% | Independent variables: extract concentrations of 0.5%, 1%, 1.5% and 2%. Bound variables: Stability of the preparation | IC50 value of matoa leaf extract 54.63 ppm (strong) All formulas stored at room temperature meet the physical stability requirements of the cream including organoleptics, homogeneity, pH and viscosity. | (92) |
6 | Herbal Drinks | Matoa Leaf Water Extract and Sirih Leaf Water Extract | Infusion | Combination of matoa leaf extract and betle leaf extract 7:3, 5:5, 3:7 | Independent variables: extract concentrations of 7:3, 5:5 and 3:7 Dependent variables: antioxidant activity, physical characteristics: color, organoleptic, taste and acceptability | The best formula is the ratio of 7:3 and the addition of 15% honey) with a total flavonoid value of 6.3278% and antioxidant activity (IC50) of 64.7259 µg/mL, with a color organoleptic value (descriptive) of 4.70 (dark brown), aroma organoleptic (hedonic) of 3.80 (somewhat like), taste organoleptic of 3.53 (somewhat like), and general acceptance of 3.95 (somewhat like). | (93) |
7 | Jelly Candy | Ethanol Extract of Matoa Fruit Bark | Maceration with 96% ethanol | 15%, 20% and 25% | Independent variables: extract concentrations of 15%, 20% and 25%. Dependent variables: moisture content, ash content, pH, and IC50 and AAI of matoa fruit peel jelly candy. | Moisture content, ash content, and pH were in accordance with the requirements of the Indonesian National Standard (SNI). Based on the IC50 and AAI values, the antioxidant activity of the three formulas is included in very strong antioxidants and moderate antioxidants. | (94) |
8 | Cream | Matoa Leaf Ethanol Extract | 96% ethanol maceration | 2% | Independent variables: 2% extract concentration and stearic acid as emulsifier with concentrations of 9%, 12%, and 15%20% and 25%. The dependent variable is the physical characteristics of the cream | The best formula in formula II based on the results of physical evaluation of preparations including organoleptic tests of brown color, distinctive aroma, soft texture, homogeneous preparation, pH 5.15 and spreadability 5.5 cm meets the requirements of evaluating cream preparations. | (95) |
9 | Cream | Matoa Leaf Ethanol Extract | 96% ethanol maceration | 0.5 g | Independent variable: amount of span 60 and tween 80 Dependent variable: Cream physical characteristics and antioxidant activity (IC50). | The best formula Tween 80: span 60 is 50%:50% with a strength of 3.31 cm, pH 5.9, adhesion 58.60 s with antioxidant activity IC50 40.6 ppm (very strong). | (96) |
10 | Effervescent Powder | Matoa Leaf Ethanol Extract | Maceration ethanol 96% | 0.5%, 0.1 % and 0.15% | Independent variable: Extracts of 0.5 g, 0.10 g and 0.15 g Dependent variable: Physical characteristics of effervescent powder (organoleptic, moisture content, pH, flow speed, flow time, froth height) and antioxidant activity (IC50). | The optimum formula is formula 3 with a moisture content of 2.89%, pH 4.26, flow speed of 5.3 s, dissolving time of 60 s, froth height of 3.5 and IC50 71.92 ppm (strong). | (97) |
11 | Liquid Soap | Esktrak Kulit batang matoa | Maceration ethanol 96% | 0.5, 1 dan 1.5 % | Antibacterial activity used the Kirby -Bauer test methode that was better known as disc diffusion | Daimeter of inhibitory zones in cultures of S. aureus F1 9.03± 0.64; F2 10.84 ± 0.65 and F3 12.16 ± 0.91. In E coli F1 7.36 ± 0.26; F2 7.60 ± 0.16 and F3 7.82 ± 0.14 The matoa’s stem bark have been effective in inhibiting the growth of S.aureus, but not effective in inhibiting the growth of E.Coli bacteria | (98) |
However, none of these additional compounds exhibited anti-HIV-1 integrase activity at a concentration of 100 µM, indicating that proanthocyanidin A2 is the primary active compound responsible for the observed antiviral activity in the extract.
Matoa Dosage Form
Various pharmaceutical dosage forms made from Matoa have been studied (see Table 10), including lotions, peel-off masks, gels, body scrubs, creams, herbal drinks, jelly candies, liquid soaps, and effervescent powders. These preparations are still considered conventional and relatively simple.
Among them, antioxidant creams made from ethanol extracts of Matoa leaves are the most widely formulated, with variations in both emulsifiers and extract concentrations. One study reported that Matoa leaf ethanol extract has an IC₅₀ value of 54.63 ppm, indicating strong antioxidant activity. When formulated at concentrations of 0.5%, 1%, 1.5%, and 2%, the resulting creams showed good physical stability in terms of organoleptic properties, homogeneity, pH, and viscosity, and remained stable when stored at room temperature (92).
In a study by Anglia et al. (95), a 2% ethanol extract was formulated with stearic acid concentrations of 9%, 12%, 15%, 20%, and 25%. The best formulation was Formula 2 (with 12% stearic acid), which met all physical evaluation parameters. Stearic acid acts as an emulsifier, contributing to the desired cream consistency and reducing the greasy sensation on the skin (99).
Another study using 0.5 g of 96% ethanol extract combined with a 50:50 mixture of Span 60 and Tween 80 emulsifiers yielded better physical characteristics compared to formulations with a single emulsifier. The optimal formula had a spreadability of 3.31 cm, a pH of 5.9, an adhesion time of 58.6 s, and exhibited very strong antioxidant activity (IC₅₀ = 40.6 ppm) (96). The combination of Span 60 (lipophilic, low HLB) and Tween 80 (hydrophilic, high HLB) created a more stable cream emulsion (99).
In lotion preparations using 1.5% and 2% Matoa leaf ethanol extract, the formulations were stable in terms of organoleptic properties, homogeneity, spreadability, and flowability. However, they were unstable in terms of pH and viscosity, which was attributed to the presence of excess triethanolamine (TEA) and stearic acid in the formulation (87).
Peel-off mask formulations using 2% of 96% ethanol extract with varying concentrations of polyvinyl alcohol (PVA: 5%–15%) as a gelling agent showed distinct differences in physical properties, indicating that PVA concentration plays a significant role in the final product's consistency (88).
In liquid soap formulations, Matoa stem bark ethanol extract at a concentration of 1.0% demonstrated effective antibacterial activity against Staphylococcus aureus, but was ineffective against E. coli (98).
Matoa gel formulations using 2%, 3%, and 4% ethanol extracts from the leaves produced gels that met all physical evaluation criteria (89). Additionally, N-hexane and ethanol extracts at concentrations of 2.5%, 5%, and 10% showed that Formula 3 (10%) had the most potent antifungal effect against Trichophyton mentagrophytes (90).
Body scrubs made with extract concentrations of 1%, 2.5%, 4%, and 5.5% demonstrated that 5.5% extract concentration provided the highest antioxidant activity (IC₅₀ = 44.49 ppm, very strong), while meeting the required physical parameters and showing no irritation (84).
Oral dosage forms such as jelly candies, herbal drinks, and effervescent powders have also been developed. These formulations exhibited strong antioxidant activity (IC₅₀ values between 50 and 100 µg/mL) and met standard physical quality parameters (84, 93, 97).
The development of Matoa in various dosage forms aims to improve its usability, enhance patient compliance, and optimize its pharmacological effects.
Conclusion
Pharmaceutical dosage forms containing Matoa that have been studied for topical use include lotions, creams, body scrubs, gel masks, and liquid soaps. For oral use, formulations have been developed in the form of effervescent powders, herbal drinks, and jelly candies.
However, based on this review, the development of Matoa formulations using nanotechnology-based delivery systems remains unexplored. These include nanoemulsions, nanostructured lipid carriers (NLCs), solid lipid nanoparticles (SLNs), liposomes, transfersomes, ethosomes, and dendrimers. Incorporating such advanced delivery systems offers significant potential to enhance bioavailability, stability, and targeted delivery of active compounds.
Furthermore, extraction methods employed in the current studies are largely limited to maceration, a simple and conventional technique. To increase efficiency, future research should explore the use of modern extraction technologies such as Ultrasound-Assisted Extraction (UAE), Microwave-Assisted Extraction (MAE), Supercritical Fluid Extraction (SFE), Pressurized Liquid Extraction (PLE)/Accelerated Solvent Extraction (ASE), Enzyme-Assisted Extraction (EAE), and Pulsed Electric Field Extraction (PEF). These methods offer advantages such as shorter extraction times, higher yield and selectivity, reduced solvent usage, improved compound stability, and enhanced process customization.
In conclusion, there remains a substantial opportunity for further research to advance the pharmaceutical and health applications of Matoa (P. pinnata). Exploring its full pharmacological potential and developing innovative dosage forms and extraction techniques could significantly expand its therapeutic use and optimise its clinical efficacy.
Abbreviations
AUC = Area Under Curve; BW= Body Weight; LC= Lethal Concentration; ALT = Alanine Aminotransferase; AST = Aspartate Aminotransferase; ABTS = 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); SOD= Super Oxide Dismutase; HLB= Hydrophyl Lipophyl Balance; PVA :Polivinyl Alcohol ; TEA = Triaethanolamine.
Declarations
Ethics Statement
Not applicable.
Data Availability
Not applicable.
Funding Information
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflicting interest.
References
- Rahmawati R, Tahir M, Amir AHW. Kandungan Senyawa Kimia dan Aktivitas Farmakologi Tanaman Matoa (Pometia pinnata J.R. Forster & J.G. Forster). As-Syifaa J Farm. 2021;13(2):108–15.
- Elidar Y, Purwati. Budidaya Tanaman Matoa (Pometia pinnata) Di Pekarangan Dan Manfaatnya Untuk Kesehatan Keluarga. Yetti Elidar & Purwati JPKPM. 2022;2(2):206–9.
- Maruli Tua, Adiwirman, Herman. Pertumbuhan Dan Perkembangan Buah Serta Kadar Gula Buah Tanaman Matoa Merah (Pometia pinnata Forst). Din Pertan. 2023;38(2):171–6.
- Putri AC, Yuliana TN, Suzery M, Aminin ALN. Total Phenolic, Flavonoid, and LC-MS Analysis of the Ethanolic Extract of Matoa (Pometia pinnata) Leaves from Kudus, Central Java, Indonesia. J Kim Sains dan Apl. 2024;26(12):477–82.
- Hajar S, Rahmah W, Maharani Putri E, Septian Ressandy S, Hamzah H. Potensi Ekstrak Buah Matoa (Pometia Pinnata) Sebagai Sumber Antioksidan: Literatur Review. J Farm Sains dan Prakt [Internet]. 2021;7(1):2579–4558. Available from: http://journal.ummgl.ac.id/index.php/pharmacy
- Aulia Bakhtra DD, Monica DK, Fajrina A, Eriadi A. Skrining Aktivitas Sitotoksik Ekstrak Etanol pada Buah Matoa (Pometia pinnata) dengan Metode Brine Shrimp Lethality Test. J Farm Higea. 2022;14(1):40.
- Elena GS, Putri MDE, Munandar NH, Putri RY. Skrining Fitokimia dan Uji Aktivitas Antibakteri Ekstrak Etanol Daun Matoa (Pometia pinnata J.R. Forst & G. Forst) Terhadap Pertumbuhan Bakteri Salmonella thypi. J Farm Klin dan Sains. 2023;2023(1):1–9.
- Putri I R, Dezi H, Irdawati, Fifendy Mades, Gustina I. Uji daya hambat ekstrak daun matoa (Pometia pinnata J.R & G.forst) terhadap pertumbuhan jamur Candida albicans secara in vitro. Serambi Biol. 2022;7(4):346–54.
- Rochaeni H, Irawan C, Hanafi, Lestari PS, Sulistiawaty L, Putri ILD. The antidiabetic and anti-microbial activity analyses in the extract of methanol, ethyl acetate, and hexane from flesh of Matoa (Pometia pinnata J. R. Forst. & G. Forst). Trends Sci. 2021;18(22).
- Sulastri L, Suratman PP, Indriaty S, Hidayati NR. Uji Daya Hambat Ekstrak Etanol Kulit Buah Matoa (Pometia pinnata J.R & G Forst) Dengan Metode Cetak Lubang Terhadap Bakteri Staphylococcus aureus. J Pharmacopolium. 2022;5(2):142–7.
- Faustina FC, Santoso F. Ekstraksi Dan Pengamatan Aktivitas Antioksidan Dan Antimikroba Dari Kulit Buah Pometia Pinnata. J Penelit Pascapanen Pertan. 2017;11(2):80.
- Risna. Uji Aktivitas Antibakteri Ekstrak Etanol Daun Matoa (Pometia pinnata J.R & G.Forst) terhadap Pertumbuhan Staphylococcus aureus dan Escherichia coli. J Keperawatan Silampari. 2023;6(2):1139–49.
- Pakaya MS, Astuti Kai J, Zuriati Uno W. Potensi Ekstrak Etanol Kulit Buah Matoa Pometia pinnata J.R Forst & G.Forst) Terhadap Bakteri Penyebab Karies Gigi. Jambura J Chem. 2021;3(2):76–83.
- Siregar HN, Rahayu YP, Nasution HM, Nasution MP. Uji Aktivitas Antibakteri Nanopartikel Ekstrak Etanol Daun Matoa (Pometia pinnata J.R. Forst & G. Forst) Terhadap Bakteri Escherichia coli. J Ris Kefarmasian Indones. 2023;5(1):24–41.
- Gabriela Welma Litaay. Aktivitas Antibakteri Ekstrak Etanol Kulit Batang Matoa (Pometia pinnata) Terhadap Pertumbuhan Staphylococcus aureus. J Telenursing [Internet]. 2023;5(2):3393–401. Available from: https://journal.ipm2kpe.or.id/index.php/JOTING/article/view/7431
- Azlin SZ, Sidoretno WM, Dewi AP. Uji Aktivitas Antibakteri Fraksi Etil Asetat Daun Matoa (Pometia Pinnata J.R & G. Forst) terhadap Staphylococcus aureus. J Farm. 2023;1(1):30–41.
- Razoki. Antioxidant and Antibacterial Activities of Ethanol Extract of Matoa (Pometia pinnata) Leaves. Journall Phaemaceutical Sci. 2023;6(2):351–7.
- Sidoretno WM. Potential of the Ethanolic Extract of Matoa Leaves (Pometia pinnata J.R. & G.Forst) against Staphylococcus aureusbacteria. JPK J Prot Kesehat. 2022;10(2):107–12.
- Kumowal S, Fatimawali F, Jayanto I. Uji Aktivitas Antibakteri Nanopartikel Ekstrak Lengkuas Putih (Alpinia galanga (L.) Willd) Terhadap Bakteri Klebsiella pneumoniae. Pharmacon. 2019;8(4):781.
- Saptowo A, Supriningrum R, Supomo S. Uji Aktivitas Antibakteri Ekstrak Kulit Batang Sekilang (Embeliaborneensis Scheff) Terhadap Bakteri Propionibacterium acnes dan Staphylococcus epidermidis. Al-Ulum J Sains Dan Teknol. 2022;7(2):93.
- Anggraini W, Nisa SC, DA RR, ZA BM. Aktivitas Antibakteri Ekstrak Etanol 96% Buah Blewah (Cucumis melo L. var. cantalupensis) terhadap pertumbuhan bakteri Escherichia coli. Pharm J Indones. 2019;5(1):61–6.
- Abad MJ, Ansuategui M, Bermejo P. Active antifungal substances from natural sources. Arkivoc. 2007;2007(7):116–45.
- Rieska Alfiah R, Khotimah S, Turnip M. Efektivitas Ekstrak Metanol Daun Sembung Rambat (Mikania micrantha Kunth) Terhadap Pertumbuhan Jamur Candida albicans. Protobiont Progr Stud Biol Fak MIPA, Univ Tanjungpura, Pontianak. 2015;4(1):52–7.
- Hong LS, Ibrahim D, Kassim J, Sulaiman S. Gallic acid: An anticandidal compound in hydrolyzable tannin extracted from the barks of Rhizophora apiculata Blume. J Appl Pharm Sci. 2011;1(6):75–9.
- Putri PA, Chatri M, Linda A, Violita. Characteristics of Saponin Secondary Metabolite Compounds in Plants Karakteristik Saponin Senyawa Metabolit Sekunder pada Tumbuhan. Serambi Biol. 2023;8(2):251–8.
- Ismaini L. Aktivitas Antifungi Ekstrak ( Centella asiatica (L. ) Urban terhadap Fungi Patogen pada Daun Anggrek ( Bulbophyllum flavidiflorum Carr. ). 2011;14(D):47–50.
- Maisarah M, Chatri M, Advinda L. Characteristics and Functions of Alkaloid Compounds as Antifungals in Plants Karakteristik dan Fungsi Senyawa Alkaloid sebagai Antifungi pada Tumbuhan. Serambi Biol. 2023;8(2):231–6.
- Lutfiyanti Rosiska, Widodo M, Eko D. Aktivitas Antijamur Senyawa Bioaktif Ekstrak Gelidium latifolium Terhadap Candida albicans. J Pengolah dan Bioteknol Has Perikan. 2012;1:1–8.
- Santi I, Putra B, Rahman FU. Uji Efek Analgesik Ekstrak Etanol Daun Matoa (Pometia pinnata J.R. Forst & G. Forst) pada Mencit Putih (Mus musculus) Jantan. J Borneo. 2023;3(2):72–9.
- Lumintang RF, Wuisan J, Wowor PM. Uji Efek Aanalgesik Ekstrak Kulit Batang Pohon Matoa (Pometia pinnata) Pada Mencit (Mus musculus). J e-Biomedik. 2015;3(2):3–8.
- Baslani CA, Marsiati H, Wuryanti S. Antioxidant Activity of Matoa Leaves (Pometia pinnata) and Sourpus Leaves (Annona muricata L.) Using DPPH Metod with Various Solvents. Med Sains J Ilm Kefarmasian. 2023;8(2):501–10.
- Islami D, Anggraini L, Wardaniati I. Aktivitas Antioksidan dan Skrining Fitokimia dari Ekstrak Daun Matoa (Pometia pinnata). J Farm Higea. 2021;13(1):30.
- Sidoretno WM, Sintiyani I. Aktivitas Antioksidan Fraksi n-hexan, Kloroform dan etil asetat Daun Matoa (Pometia pinnata J.R & G. Forst) Terhadap DPPH (2,2-difenil-1-pikrilhidrazil). JOPS (Journal Pharm Sci. 2018;2(1):36–40.
- Nabilah A, Sutoyo S. Uji Aktivitas Antioksidan Ekstrak Metanol Kulit Batang Tumbuhan Matoa (Pometia pinnata). UNESA J Chem [Internet]. 2019;8(3):117–8. Available from: https://jurnalmahasiswa.unesa.ac.id/index.php/unesa-journal-of-chemistry/article/view/30911
- Wulandari L, Nugraha AS, Himmah UA. Penentuan Aktivitas Antioksidan dan Antidiabetes Ekstrak Daun Matoa (Pometia pinnata J.R. Forst. & G. Forst.) secara In Vitro. J Kefarmasian Indones. 2021;11(2):132–41.
- Djabar TO. Uji Aktivitas Antioksidan Ekstrak Etanol 70% Daun Matoa (Pometia pinnata) Terhadap Aktivitas ALT Dan AST Darah Tikus Putih Yang Diinduksi Karbon Tetraklorida (CCl4). Indones J Heal Sci. 2021;1(1):10–5.
- Poli AR, Katja DG, Aritonang HF. Potensi Antioksidan Ekstrak dari Kulit Biji Matoa (Pometia pinnata J. R & G. Forst). Chem Prog. 2022;Vol. 15. N(1):25–30.
- Irawan C, Rochaeni H, Sri Lestari P, Poppy Sri Lestari C, Sulistiawaty L. Evaluation of DPPH free radical scavenging activity of Pometia pinnata from Indonesia. Journal. 2017;6(8):403–6.
- Maulina D. Pengaruh Pemberian Ekstrak Etanol 70% Daun Matoa (Pometia pinnata) Terhadap Kadar Superoxide Dismutase (SOD) Hati Tikus. Indones J Heal Sci. 2021;1(1):1–3.
- Kuspradini H, Pasedan WF, Kusuma IW. Aktivitas Antioksidan dan Antibakteri Ekstrak Daun Pometia pinnata. J Jamu Indones. 2016;1(1):26–34.
- Martiningsih NW, Widana GAB, Kristiyanti PLP. Skrining Fitokimia Dan Uji Aktivitas Antioksidan Ekstrak Etanol Daun Matoa (Pometia pinnata) dengan Metode DPPH. In: Prosiding Seminar Nasional MIPA 2016 Universitas Pendidikan Ganesha. 2016. p. 332–8.
- Fitri A, Andriani M, Sudarman A, Toharmat T, Yonekurac L, Tamura H, et al. Screening of antioxidant activities and their bioavailability of tropical fruit byproducts from Indonesia. Int J Pharm Pharm Sci. 2016;8(6):96–100.
- Sulistiani, Nurillahi F L, Susana L. Pengukuran aktivitas antioksidan ekstrak etanol buah matoa (Pometia pinnata) dengan metode DPPH (2,2-difenil-1,1 pikrilhidrazil). 2024;8(2):50–60.
- Rumagit HM, Runtuwene MR, Sudewi S. Uji Fitokimia Dan Uji Aktivitas Antioksidan Dari Ekstrak Etanol Spons Lamellodysidea herbacea. PHARMACON J Ilm Farm. 2015;4(3):183–92.
- Ambarsari N, Dayanti R. Literature Review: Aktivitas Antioksidan Ekstrak dan Fraksi Daun Matoa (Pometia pinnata). J Syifa Sci Clin Res. 2024;5(3):447–52.
- Mahmud NRA, Muhammad N, Nurhalifah. Effect of the Adding of Natural Antioxidant in the Stored Traditional Coconut Oil on the Free Fatty Acid Value. Proc Internat Conf Sci Engin. 2021;4(February):76–9.
- Ayu Rismiasih AKJ. Uji Aktivitas Tabir Surya Ekstrak Etanol Daun Matoa (Pometia pinnata) secara in vitro. J Komunitas Farm Nas. 2022;2(1):213–5.
- Kurnianto E, Rahman IR. Potensi Tabir Surya Ekstrak Etanol Daun Matoa (Pometia pinnata) Dengan Variasi Konsentrasi Pelarut. J Ilm Ibnu Sina Ilmu Farm dan Kesehat. 2021;6(2):102–8.
- Furi M, Feriansyah R, Fadhli H, Utami R, Lestari P. Uji Aktivitas Antioksidan Dan Tabir Surya Ekstrak Etanol Dan Fraksi Daun Terap (Artocarpus odoratissimus Blanco). JFIOnline | Print ISSN 1412-1107 | e-ISSN 2355-696X. 2023;15(2):196–205.
- Furi M, Ainun Alfatma, Rahma Dona, Armon Fernando, Fina Aryani, Rahayu Utami, et al. Uji Ihibitor Enzim Tirosinase Ekstrak Dan Fraksi Daun Kedabu (Sonneratia ovata Backer) Secara In-Vitro. J Ilm Manuntung. 2022;8(2):201–14.
- Sauriasari R, Azizah N, Basah K. Tyrosinase inhibition, 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity, and phytochemical screening of fractions and ethanol extract from leaves and stem bark of matoa (Pometia pinnata). Asian J Pharm Clin Res. 2017;10(Special Issue October):85–9.
- Sholikha M, Febriani A, Wahyuningrum A. Formulasi gel ekstrak lobak (Raphanus sativus l .) sebagai antioksidan dan inhibitor tirosinase. J Ilmu Kefarmasian [Internet]. 2020;13(1):15–20. Available from: https://doi.org/10.35311/jmpi.v8i2.248
- Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: Biology and development. Postep Dermatologii i Alergol. 2013;30(1):30–41.
- Mustika R, Hindun S, Auliasari N. Potensi Tanaman Sebagai Pencerah Wajah Alami. J Sains dan Kesehat. 2020;2(4):558–62.
- Afiah N, Santi I, Putra B. Dose Optimization of Antihyperuricemia Effects of Matoa Leaf (Pometia pinnata J.R.Forst & G.Forst) in Rats. Pharm Reports. 2023;2(2):10–3.
- Latief M, Tarigan IL, Sari PM, Aurora FE. Antihyperuricemia Activity of Ethanol Extract of Sungkai Leaves- (Peronema canescens Jack) in Male White Mice. Pharmacon J Farm Indones. 2021;18(1):23–37.
- Nurihardiyanti, Yuliet, Ihwan. Aktivitas Diuretik Kombinasi Ekstrak Biji Pepaya (Carica papaya L) Dan Biji Salak (Salacca zalacca varietas zalacca (Gaert.)Voss) Pada Tikus Jantan Galur Wistar (Rattus norvegicus L). J Farm Galen (Galenika J Pharmacy). 2015;1(2):105–12.
- Sundhani E, Syarifah DCN, Zumrohani LR, Nurulita NA. Efektivitas Ekstrak Etanol Daun Adam Hawa (Rhoeo discolor) Dan Daun Pucuk Merah (Syzygium campanulatum Korth.) Dalam Menurukan Kadar Gula Darah Pada Tikus Putih Jantan Galur Wistar Dengan Pembebanan Glukosa. Pharmacy. 2016;13(2):137–49.
- Mariyo Jane Sanggel P. Efek Antihipergklikemi dan perbaikan fungsi ginjal, ekstrak etanol daun matoa (Pometia Pinnata J.R & G.Forst) dengan parameter kadar kreatinin dan histopatologi pada tikus diabetes nefropati yang diinduksi streptozotosin-nikotinamid. SUPLEMEN. 2023;Volume 15.
- Wahyuni AS, Muflihah CH, Fadhilah A, Oksaputra AK, Ningrum NFS, Bakhtiar M. Antidiabetic activity of matoa leaves (Pometia pinnataJ.R.Forst & G. Forst) extract on hyperglycaemic alloxan-induced rats. Indones J Pharm Sci Technol. 2023;10(3):119.
- Dangeubun, E.J & Katja D. Sifat Toksisitas dan Kemampuan Penghambatan Enzim Α-Amilase Dari Ekstrak Biji Buah Matoa (Pometia pinnata J. R & G. Forst). Chem Prog. 2022;15(1):1–8.
- Sukiman M, Margaretha JA, Irawan C, Sulistiawaty H, Sulistiawaty L. Evaluation of antidiabetes activity of matoa seed extract (Pometia pinnata) using enzym α-glucosidase. Pharma Innov J [Internet]. 2018;7(5):10–2. Available from: www.thepharmajournal.com
- Sumarlin LO, Suprayogi A, Rahminiwati M, Satyaningtijas A, Nugraha AT, Sukandar D, et al. Identification of Compounds Flavonoids Namnam Leaf Extract (Cynometra cauliflora) As Inhibiting A-Glucosidase. J Phys Conf Ser. 2020;1594(1).
- Larantukan SVM, Setiasih LNE, Widyastuti SK, et al. Pemberian Ekstrak Etanol Kulit Batang Kelor Glukosa Darah Tikus Hiperglikemia. Indones Med Veterinus. 2014;3(4):292–9.
- Hasan H, Ain Thomas N, Hiola F, Nuzul Ramadhani F, Ibrahim AS. Skrining Fitokimia dan Uji Aktivitas Antioksidan Kulit Batang Matoa (Pometia pinnata) Dengan Metode 1,1-Diphenyl-2 picrylhidrazyl (DPPH). Indones J Pharm Educ. 2022;2(1):67–73.
- Turnip NUMB, Panjaitan YL. Activity Test Ethanol Extract Matoa (Pometia pinnata) Antinflammation Against Male Rats Induced Caragenan. J Farm. 2022;5(1):57–61.
- Parlin DA, Nasution M., Nasution H., Daulay AS. Skrining Fitokimia dan Uji Sitotoksisitas Ekstrak Etanol Daun Matoa (Pometia pinnata) dengan Metode BSLT. Farmasainkes J Farm Sains, dan Kesehat. 2022;2(1):38–48.
- Hasim H, Arifin YY, Andrianto D, Faridah DN. Ekstrak Etanol Daun Belimbing Wuluh (Averrhoa bilimbi) sebagai Antioksidan dan Antiinflamasi. J Apl Teknol Pangan. 2019;8(3):86.
- Purwidyaningrum I, Sukandar EY, Fidrianny I. Diuretic activity of matoa leaves extracts and fractions (Pometia pinnata J.R. Forster) and its influence on potassium and sodium levels. Asian J Pharm Clin Res. 2017;10(Special Issue may):31–4.
- Lepangkari JS, Elisa N, Seran IC. Uji Aktivitas Antihipertensi Ekstrak Etanol Daun Matoa (Pometia pinnata J.R. Forster & G. Forster) pada Tikus Jantan yang Diinduksi Angiotensin II dengan Parameter Blood Flow. Publ Penelit Terap dan Kebijak. 2023;6(1):65–72.
- Elisa N, Xaverius F, Wibowo S. Hypertension Profile of Angiotensin Receptor Blocker From Matoa Leaves Extract ( Pometia pinnata J . R . Foster & G . Foster ) In Angiotensin II Induced-Male Rat. Str J Ilm Kesehat. 2020;9(2):1830–6.
- Purwidyaningrum I, Peranginangin JM, Sahira I. The influence of extracts and fractions from matoa leaves (Pometia pinnata) on angiotensin I levels. Pharm Educ. 2021;21(2):168–71.
- Oentari OD, Peranginangin JM, Purwidyaningrum I. Activity Test of Matoa Leaves on Angiotensin II as an Increasing SOD and GPx. :54–9.
- Marunaka Y, Marunaka R, Sun H, Yamamoto T, Kanamura N, Inui T, et al. Actions of quercetin, a polyphenol, on blood pressure. Molecules. 2017;22(2):5–8.
- Suedee A, Tewtrakul S, Panichayupakaranant P. Anti-HIV-1 integrase compound from Pometia pinnata leaves. Pharm Biol. 2013;51(10):1256–61.
- Purwidyaningrum I, Dzakiwan M. Uji Aktivitas Diuretik Ekstrak Daun Matoa (Pometia pinnata) pada Tikus Jantan Galur Wistar. J Farm Indones. 2015;Vol. 12 No(ISSN: 1693-8615):79–84.
- Melendez-Camargo ME, Contreras-León I, Silva-Torres R. Diuretic effect of alkaloids fraction extracted from Selaginella lepidophylla (Hook. et Grev.) Spring. Bol Latinoam y del Caribe Plantas Med y Aromat. 2014;13(1):92–9.
- Nurihardiyanti. Review artikel : Aktifitas Antihiperurisemia Beberapa Tanaman Indonesia. Farmaka Suplemen. 2013;15(2):11–22.
- Khu A, Syahputra RA, Lie S, Nugraha SE, Situmorang PC. Amelioration of cisplatin-induced kidney injury by Pometia pinnata. Pharmacogn J. 2021;13(5):1257–68.
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene [Internet]. 2012;31(15):1869–83. Available from: http://dx.doi.org/10.1038/onc.2011.384
- Nuryadin ZD, Pebiansyah A, Aprilia AY. Aktivitas Nefroprotektif Ekstrak Etanol Bunga Telang (Clitoria ternatea L.) Terhadap Tikus Yang Diinduksi Parasetamol. Pharmacoscript. 2021;4(2):173–80.
- Santi I, Putra B, Kosman R, Ramadhani FA. Uji Efek Antidiare Ekstrak Etanol Daun Matoa (Pometia pinnata J.R Forst& G.Forst ) on Male White Rats ( Rattus novergicus ). 2023;15(2):151–7. Available from: https://jurnal.farmasi.umi.ac.id/index.php/as-syifaa/article/view/99
- Hasanah F, Saputri M, Siahaan DN, Kusuma PA, Matondang SRR. Uji Antidiare Ekstrak Etanol Daun Matoa (Pometia pinnata) Terhadap Mencit Jantan Diinduksi Oleum Ricini. J Pharm Heal Res. 2023;4(2):342–8.
- Islami D, Ramadhan W, Iballa BDM, Siregar ED. Skrining Fitokimia Dan Uji Aktivitas Antioksidan Dari Ekstrak Kulit Buah Matoa Pometia pinnata J.R. Forst. & G. Forst. J Aisyiyah Med. 2024;9(1):91–101.
- Wahyuni HS, Marianne, Nugraha SE, Sumantri IB, Jap CC, Hijriyan TB. Unveiling the anticancer potential of the ethanolic extract from Pometia pinnata: Molecular dynamics targeting CHK1 and cytotoxicity study on MCF-7 cells. Pharmacia. 2024;71:1–15.
- Suzuki T, Nagata M, Kagawa N, Takano S, Nahrowi, Nomura J. Anti-obesity effects of matoa (Pometia pinnata) fruit peel powder in high-fat diet-fed rats. Molecules. 2021;26(21).
- Riyanti S, Sulastri L, Rizikiyan Y, Prayoga IB. Formulasi Dan Uji Stabilitas Lotion Ekstrak Kulit Buah Matoa (Pometia pinnata J.R & G. Forst) Konsentrasi 1,5% Dan 2%. Medimuh J Kesehat Muhammadiyah. 2022;3(1):11–20.
- Febriyanto Y, Dwiningsih A. Formulasi Gel Peel Off Ekstrak Etanol Daun Matoa (Pometia pinnata J.R. Forst & G. Forst) dengan Variasi Konsentrasi PVA sebagai Gelling Agent. J Farm Sains. 2020;3(1):1–5.
- Utama VK, Hendrika Y, Astuti F. Gel Preparation Using Matoa Leaf (Pometia pinnata) Ethanol Extract: Formulation and Physical Evaluation. JPK J Prot Kesehat. 2022;11(1):46–51.
- Ayu TZM, Veranita W, Septiarini, Dwi A. Evaluasi Sediaan Gel Ekstrak n -Heksan Dan Etanol Daun Matoa (Pometia pinnata J.R.G.Forst) Terhadap Pertumbuhan Jamur Trichophyton mentagrophytes ATCC 9533. War Bhakti Husada Mulia J Kesehat [Internet]. 2023;10(1):1–19. Available from: https://jurnal.stikes-bhm.ac.id/index.php/jurkes/article/view/350
- Hehakaya MO, Edy HJ, Siampa JP. Formulasi dan Uji Aktivitas Antioksidan Sediaan Body Scrub Ekstrak Etanol Daun Matoa (Pometia pinnata). Pharmacon [Internet]. 2022;11(4):1778–85. Available from: https://ejournal.unsrat.ac.id/v3/index.php/pharmacon/article/view/42148/40373
- Tahalele E. Formulasi Sediaan Kosmetik Krim Dari Ekstrak Daun Matoa (Pometia pinnata) Dan Uji Aktivitas Antioksidan. Indones Nat Res Pharm J. 2018;3(2):2502–8421.
- Rizkah Syarazani M IS, Ginting S. Pengaruh Perbandingan Ekstrak Daun Matoa (Pomentia pinnata ) Dengan Ekstrak Daun Sirih (Piper betle) Dengan Penambahan Madu Terhadap Mutu Minuman Herbal. J Food Technol Agroindustry. 2024;6(1).
- Fadilla IN, Gunawan E, Pratiwi RD, Simaremare ES. Pengembangan Produk Permen Jelly Kulit Buah Matoa (Pometia pinnata) Asal Papua Sebagai Antioksidan. J Darma Agung [Internet]. 2023;31(Oktober):126–36. Available from: https://jurnal.darmaagung.ac.id/index.php/jurnaluda/article/view/3724
- Anglia R, Barus R, Saputra CT, Liambo IS, Ilyas M. Formulasi Sediaan Krim Dari Ekstrak Etanol Daun Matoa (Pometia pinnata). Pharmauho J Farm Sains, dan Kesehat. 2022;8(1):38–42.
- Restuinjaya LA, Simaremare ES, Pratiwi RD. Optimization of Tween 80 and Span 60 on Cream Ethanol Extract the Leaves Matoa (Pometia Pinnata) as an Antioxidant. J Adv Pharm Pract [Internet]. 2019;1(2):11–21. Available from: http://doi.org/10.5281/zenodo.3247411
- Pamangin YC, Pratiwi RD, Dirgantara S, Fakultas PF, Uncen M, Kampus J, et al. Pemanfaatan Limbah Kulit Buah Matoa (Pometia Pinnata) Asal Papua Menjadi Minuman Effervescent Yang Berantioksidan Tinggi. J Kim [Internet]. 2020;4(1):52–62. Available from: https://ejournal.uncen.ac.id/index.php/JA/article/view/1172/986
- Zeiniyah S, Susanty Simaremare E. Antibacterial Activity Test of the Liquid Soap of Matoa’s Stem Bark (Pometia pinnata) against Staphylococcus aureus and Escherichia coli. Recent Trends Pharm Sci Res [Internet]. 2019;1(2):40–6. Available from: http://doi.org/10.5281/zenodo.3247351
- Wahyudi MDP, Astari NKE, Adnyani NPRM, Ayu DA, La EOJ. Optimasi Komposisi span 60 Dan Tween 80 Dalam Sediaan Body Cream Ekstrak Umbi Bit Menggunakan Metode Simplex Lattice Design. J Farmasetis. 2023;12(4):403–12.

 ETFLIN
Notification
ETFLIN
Notification






