Sterility of Ophthalmic Solutions as a Factor in the Evolution of Primary Packaging for Eye Drops: A Literature Review
by Ivan Sergeevich Ivanov ★ , Diana Alexandrovna Akhmedova , Yulia Anatolyevna Koroleva , Denis Olegovich Shatalov
Academic editor: Adeleye Ademola Olutayo
Sciences of Pharmacy 3(1): 40-50 (2024); https://doi.org/10.58920/sciphar0301211
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
10 Jan 2024
17 Feb 2024
18 Feb 2024
25 Feb 2024
Abstract: Preservatives in eye drops, while not always necessary, can lead to undesirable effects. Developing preservative-free solutions demands special measures for sterility, utilizing multidose or monodose primary packaging. This review explores the merits and drawbacks of these packaging types. A literature search on PubMed, Google Scholar, and EMBASE until December 2023, using MESH terms, yielded 28 studies on multidose ampoules and 24 on monodose packaging. Heterogeneous data revealed advantages and disadvantages concerning patient use and manufacturing. Chronologically presenting the development of eye drop packaging, this study finds Droptainer® simple but unable to maintain sterility. Comod®, 3K®, ABAK®, Novelia®, and Ophthalmic Squeeze Dispenser show high sterility probability, with Comod® and ABAK® having a contamination risk. Novelia® excels with long-term sterility and better control. Ophthalmic Squeeze Dispenser, FDA-approved, boasts a smaller carbon footprint. Unit-dose systems preserve sterility and offer design flexibility. Proposing an alternative, blister technology maintains sterility, is convenient and safe, and holds promise for recycling. This comprehensive assessment aids in understanding the evolving landscape of eye drop packaging, emphasizing the importance of sterility, convenience, and environmental impact.
Keywords: SterilityPreservativeMultidose ampouleUnit-doseBlow-fill-sealDosepan blister
Introduction
The ophthalmic drug market, valued at $16.2 billion, plays a significant role in driving growth within the healthcare industry (1). Eye drops account for 89% of all registered ophthalmic drugs (2). This is largely due to their non-invasive nature and ease of access to various segments of the eye, making them a preferred method of treatment for ophthalmic diseases (3). A significant number of patients require daily, long-term use of eye drops to manage their conditions (4, 5). Other conditions such as hay fever and glaucoma also require the long-term use of eye drops. All eye drops must be free from pathogenic microflora, as microbial contamination poses a significant risk factor for developing bacterial keratitis (6-11). Patients who have recently undergone surgery and those using topical steroids, which reduce the immune properties of the optic organ, are at a higher risk of infection (12). The need for sterility was initially recorded in the United States Pharmacopeia (USP) in 1955, even though the standards for aseptic production of ophthalmic products were established earlier in 1947 by R. Alexander and W. Conner (13).
Eye drop formulations that are packaged under sterile conditions have the potential to become contaminated after they are opened, and using contaminated eye drops increases the risk of ocular infections (14). Contamination typically occurs due to improper handling, such as directly touching the tip with one's fingers, which can introduce pathogens into the vial (15). Additionally, ambient air entering the eye drops can also contaminate the contents. To maintain the sterility of the composition, preservatives are added. Thiomersal was commonly used in the 1960s but was replaced with benzalkonium chloride due to its toxicity (16). Polyquaternium-one has also proven to be effective in numerous tests. Other preservatives include polyhexamethylene biguanide, sodium perborate, Purite® (Allergan, Dublin, Ireland), and Sofzia® (Alcon Inc., Fort Worth, TX, USA) (17, 18). A multicenter study involving 9658 patients who used eye drops with and without preservatives showed that those who used eye drops without preservatives had significantly fewer ocular symptoms and signs of irritation compared to those who used eye drops with preservatives (19). Overall, patients and clinicians prefer using products without preservatives (20). Hence, an affordable and user-friendly packaging system that ensures product sterility is the preferred solution. This review explores the merits and disadvantages of these packaging types. The information presented in this review will enable the reader to understand how primary packaging for ophthalmic medications has evolved, identifying the driving factors behind these changes. Additionally, it introduces the possibility of utilizing blister packaging not only for solid dosage forms but also for eye drops. This innovation could potentially mark the next phase in the development of packaging within the eye pharmaceutical industry.
Methodology
PubMed, Google Scholar, and EMBASE databases were systematically checked to survey literature about the primary packaging of eye drops. Articles from all years were considered, and the search incorporated specific keywords such as "ophthalmic drug market," "preservatives," "bacterial contamination," "sterility," "primary packaging," "drop volume," "actuation force," "drug distribution," "Blow-Fill-Seal," "production costs," "blister packaging," "eye care," and "recycling" in various combinations. Inclusion criteria encompassed peer-reviewed articles that not only addressed the patient use of eye drops within a given primary packaging but also contributed insights into the sustainability of the packaging in its production. Additionally, references within identified articles were meticulously screened for relevance and included in the narrative review when pertinent to the overarching theme.
Main part
Multidose ampoules
Multidose eye drop packaging is designed to hold multiple doses of medication in one container. According to the European Pharmacopoeia, multidose eye drop packs consist of a plastic or glass container with a screw-on or snap-on lid. Glass containers have been used for preparing medication since the 16th century when Paracelsus first mentioned them (21). Glass containers offer several advantages, including strength, resistance to washout, and blocking of most ultraviolet radiation. However, these advantages may not be enough to prevent degradation of the contents of the container (22). Glass containers also have some disadvantages. The British Pharmacopoeia and The USP require glass containers to have high chemical resistance, which is typically achieved by adding boron and aluminium (Al) oxides. Unfortunately, this increases the likelihood of Al contamination (23). Other considerations include the possibility of glass interaction with the buffer, delamination, and breakage (24-26). Differences in the glass vial manufacturing process, the nature of the dosage form, the presence or absence of an ammonium sulfate coating, storage time, and conditions, can all contribute to the likelihood of glass breakage. Additionally, glass containers are fragile and have a significant weight (27).
Since the discovery of the natural rubber polymerization method by C. Goodyear in 1839, rubber has become a viable material for producing bottles for eye drops, including corks and membranes for metered installations (28). Glass pipette tips also emerged as a viable option for production later on. However, the combination of a pipette and a bottle of eye drops proved to be a breeding ground for bacterial contamination. This issue led to a nosocomial infection in a Birmingham hospital in the 1950s and ultimately resulted in the replacement of this container design (29). As a result, glass vials with dosing pipettes are not commonly used today (13).
In 1953, Alcon Research, Ltd introduced the Droptainer® (Alcon Research, Ltd, Sinking Spring, PA, USA) eye drop bottle, which was a low-density polyethylene (LDPE) bottle with a built-in "dropper" in the neck (18, 30). The Droptainer® has become the industry standard for many eye care products. However, it has been discovered that the physical properties of bottles can vary. When comparing international brands to those from the Kingdom of Saudi Arabia, it was found that the former contained more eye drops than indicated on the bottle, while the two local brands contained less. Additionally, the diameters of the vial tips differed significantly. A patient survey revealed that local brand vials were easier to squeeze, which is an important factor for users who struggle with compression force (31, 32). Changing the type of vial used can result in a decrease in the dosed volume, which has both economic and biopharmaceutical implications. Eye drops are typically dosed between 25 μL to 70 μL, with the drop volume sometimes being reduced to 20 μL due to the small capacity of the precorneal region. In addition, the risk of drug absorption through the nasal mucosa can be high, resulting in drops as small as 5-15 μL. The dropper tip with an inserted glass capillary has made these small doses possible (33). Despite these findings, maintaining sterility remains a critical issue for multidose ampoules.
In the early 1990s, Europe saw the invention of dosing systems for eye drops that could maintain the sterility of the drug Comod® (Ursa Pharm, Arzneimittel, Germany) and the similar 3K® system (Aero Pump, Hochheim-nah, Germany) (34). The Comod® system uses an elastic inner bag containing the liquid and is surrounded by an air space. After dosing, the air returns to this space without touching the liquid, while the next dose of the drug stays in the spout. The Comod® design employs a multi-component mechanical drop nozzle, starting with a ball valve at the bottom of the dropper, followed by a spring and a fluid channel. The bottle nozzle is also sealed with a spring and a piston. When the piston is compressed, liquid flows out through the side capillary, while the piston itself prevents the liquid from flowing back to prevent contamination. The negative pressure of the ball valve draws the next dose into the nozzle after the current dose is instilled. However, Matthias Birkhoff et al. discovered that if the outer part of the Comod®-type vial spout is infected with Pseudomonas aeruginosa culture, the resulting drop may also be contaminated (35). The silver rod used in the design, located near the outlet tip, did not contribute to the elimination of the pathogen by the oligodynamic effect (36). In contrast, another study showed no evidence of cross-contamination in any Comod® system bottle, suggesting the possibility of preventing pathogens from entering preservative-free eye drops (37). The 3K® system is similar to the Comod® system, with the liquid passing through a sealed valve that prevents backflow, thereby releasing the chamber to be replenished with the next dose of the drug. Unlike the Comod® system, the dosed product is compensated by the airflow passing through a filter. This system also includes a silver coil near the outlet to provide additional antimicrobial properties (18). One potential drawback of these systems is the difficulty that arises regarding patient compliance. The actuation force required for such vials is 25 –28 Newton (N), which is much higher than that of standard vials (7–10 N) (34). In a study of 13 multidose and single-use eye drop dispensers, the authors determined that the force required to extract the drop varied significantly depending on the dropper's design, ranging from 6.4-23.4 N. This aspect can significantly affect compliance with the treatment regimen and its effectiveness in the patient (38).
In 1989, Thea Laboratories introduced the first generation of the ABAK® system (Thea Laboratories, Clermont-Ferrand, France). ABAK® works by filtering the formulation containing the preservative through a microporous pad, which removes the preservative by adsorbing it onto the pad's porous surface before installation. However, a disadvantage of this solution was the risk of some active ingredients, such as timolol, being adsorbed (39). Early ABAK® systems featured a silver mesh design around the dispensing port. The system was gradually improved until the third generation of ABAK® was introduced in 2005. The current version uses sterile filtration of eye drops through a special microporous pad and a hydrophilic polyethersulfone membrane with a pore size of 0.2 μm. The membrane is made hydrophobic by a special surface treatment, allowing liquid to flow out of the vial but not allowing it to return, thus removing contaminating particles from the air and returning to the vial. The new generation of ABAK® also solved the problem of adsorption of some components, such as timolol (40). Therefore, in the current version of the ABAK® bottle, the formulation should not contain traces of preservatives. The volume of a single dose is 30 μL. However, the porous gasket and hydrophilic membrane create significant resistance in the path of the drug flow, resulting in a fixed actuation force in the range of 17-20 N, which is lower than that of the Comod® and 3K® systems (41). One disadvantage of this technology is that the gasket and membrane structures may limit the use of low-viscosity formulations. Nevertheless, the possibility of dosing a 2% solution of polyvinylpyrrolidone through an ABAK® vial has been demonstrated (42, 43). It should be noted that the ABAK® system vial is susceptible to high altitudes, which can result in uneven droplet outflow, leakage, or obstruction of uniform dosing when used in flight (18, 34). Furthermore, Alexandre Xavier da Costa et al. demonstrated the risk of droplet contamination when the outer part of the nozzle is contaminated with Pseudomonas aeruginosa (44).
In 2010, Nemera La Verpilliére introduced the Novelia® system (Nemera La Verpilliére, La Verpillière, France), which allows for droplet sizes ranging from 28 μL to 46 μL. The Novelia® bottle has a valve mechanism based on a silicone tube, and the container is ventilated by air diffusion through a silicone membrane. Once a drop is dispensed, the outlet valve closes immediately to prevent any contaminated liquid or air from entering back through the droplet mechanism. The plastic material of the actuator, protective cap, and silicone valve contain silver ions to ensure microbial integrity (34). The required actuation force for this vial is similar to other droppers on the market, although no specific value is given, and may increase over time due to the volume being replaced by air. Kemal Ozulken et al. found no evidence of bacterial contamination during their 60-day study of the Novelia® system (45). Additionally, Kai Kaarniranta et al. showed that the Novelia® vial had better eye drop control compared to the 3K® system, possibly due to the blue tip which serves as a focus point for patients. Patients generally preferred Novelia® over 3K® because it was significantly easier to open, squeeze, position, handle, and remove residual drops from the tip (46). Based on these studies, it can be inferred that the actuation force of the Novelia® vial is less than 25 N. Another study demonstrated that the Novelia® container maintains the physicochemical and microbiological stability of the drug on par with amber glass containers and classic high-density polyethylene containers (47). Furthermore, Novelia® vials can reduce the risk of contamination of eye drops used in clinical settings, and safe use of such eye drops is possible for up to two weeks (48).
In 2011, Aptar Pharma developed the Ophthalmic Squeeze Dispenser (OSD) (Aptar Pharma, Santiago de Querétaro, Mexico) that features a spring-loaded tip seal to keep the system closed until a certain pressure is reached, at which point the formulation is forced through the orifice. As the pressure drops, the tip seal closes the hole immediately, making the return flow of contaminated liquid impossible. The air required to equalize the pressure inside the container after dosing is filtered using a filter with a 0.2 μm pore size. Although the snap-top closure of the top of the bottle on the reservoir is intended to provide a tight connection, the OSD has been reported to be inferior to the Novelia® system in several parameters mainly related to compliance, such as bottle cap snap-on, play, and increased compression force (49). Unlike other systems, the OSD liquid is not filtered and does not come into contact with metal parts. Currently, a large number of products are supplied in OSD packaging. Additionally, in 2016, the Food and Drug Administration approved the first prescription drug in such packaging (18). A manufacturer's study has also shown that reprocessing a 10 ml OSD creates a significantly smaller carbon dioxide footprint than the equivalent in single-use eye drop dispensers (34).
Single-use eye drop dispensers
Before 1950, little attention was given to errors in treatment. To reduce the frequency of errors in patient treatment and make the process of providing care as cost-effective as possible, the Unit Dose Drug Distribution (UDD) system was introduced in US hospitals in the early 1960s (50). In 1972, the General Accounting Office published a study showing that UDD contributed to safer and better patient care by minimizing medication errors. This led the American Society of Hospital Pharmacists to strongly recommend the use of UDD in hospitals and other medical institutions, which was approved by the board of directors in 1975. In 1977, the Joint Commission on Hospital Accreditation also recommended the use of UDD in hospitals (51). According to a comparative analysis in 2013, multidose ophthalmic drugs accounted for only 24% of the market (1).
The prototype for one of the first unit-dose liquid medicine containers was patented in 1938 by R.P. Scherer (52). While Droptainer® is considered the standard for multidose ampoules, Blow-Fill-Seal (BFS) droppers are the go-to for single-dose formulations. Rommelag developed BFS (Rommelag, Sulzbach-Laufen, Germany) in the early 1960s (53-55). Generally, Figure 1 presents the chronological development of the primary packaging for eye drops, detailing its internal construction.
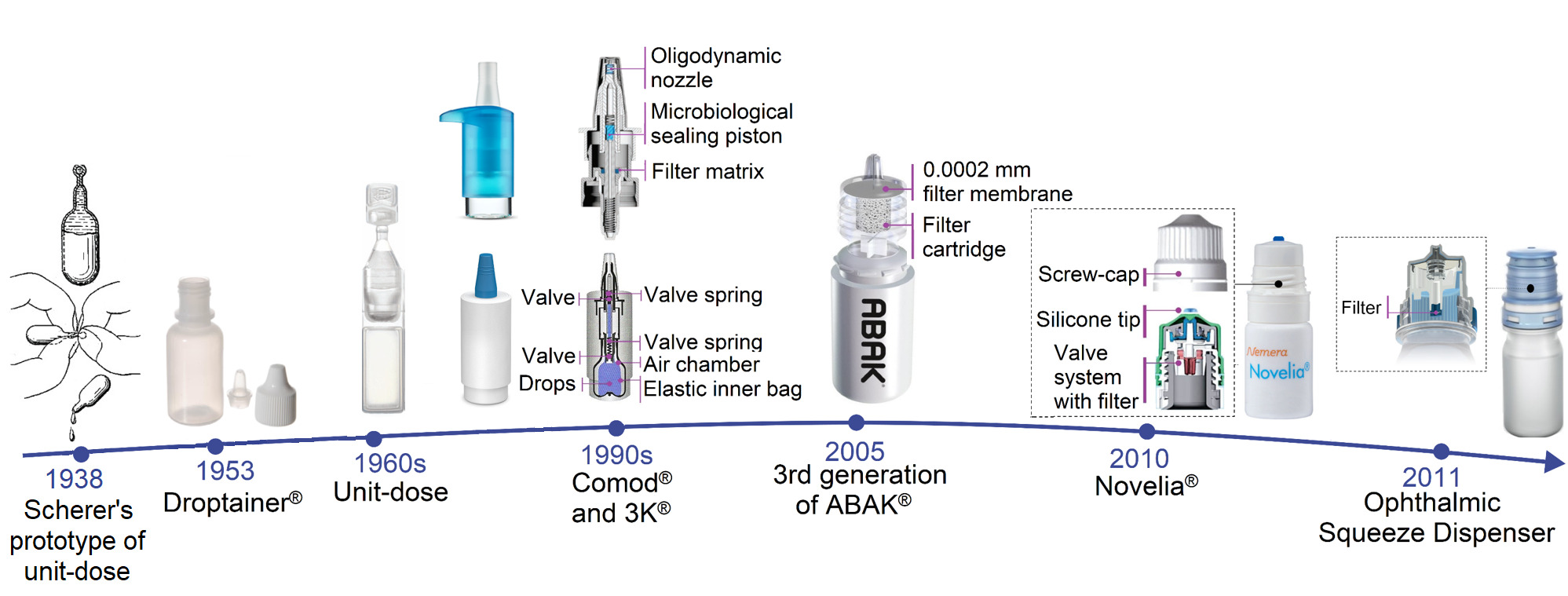
Figure 1. The chronological development of the primary packaging for eye drops.
Today, BFS is recognized by USP. In this process, polymer containers are created, filled, and sealed in a single cycle that takes around 10 seconds, with minimal operator intervention during filling (56). The primary packaging material is a granular polymer, typically LDPE or poly(propylene-co-ethylene). Plastic pellets are fed through a rotating extruder screw, where the polymer is heated to over 170 °C and subjected to pressure of over 200 bar to melt. This molten material is then squeezed out through a hole to form an oblong tube (parison). To prevent breakage of the preform, a stream of sterile filtered air is forced through the extruder die. The container is shaped either by vacuum inside the mold or by blowing air. Once molded, the blank is cut off and transferred to the filling stage, where filling takes place under a constant stream of sterile filtered air. Finally, the upper part of the mold is closed to seal the container (56, 57).
The BFS packaging design offers flexibility and low production costs. However, some data suggests that BFS is significantly more expensive than multidose ampoules for equivalent eye drops, potentially due to overfilling each unit dose with the drug (58-60). The main advantage of BFS is its sterility, which is achieved during production and can be maintained until the first opening (58). According to EU (European Union) guidelines, the filling area of a BFS machine is classified as a Class A environment with microorganism concentrations corresponding to below one colony-forming unit/m3 (CFU/m3) of air (61). Airborne contamination risks in aseptic processes depend on various factors, including the level of contaminants, air movement, and the product's nature (62). The use of KleenKut® technology (Weiler Engineering, Inc., Elgin, IL, USA) has addressed non-viable particle formation resulting from the contact of an electrically heated cut-off knife with a molten workpiece (63, 64). This technology uses ultrasound instead of a hot-wire shutoff to prevent smoke formation, which needs to be eliminated from the system with an air extractor. Many studies have contributed to optimizing the design and operating conditions of BFS machines (65-67). Despite these optimizations, some drawbacks of BFS technology include its high degree of permeability associated with the material used, which can interact with the drug components. Local heating of the liquid in the BFS can also be problematic for thermosensitive preparations, but the technology has been upgraded to minimize this effect (58, 68-72).
When it comes to patient compliance, the unit-dose packaging format may pose difficulties for people with disabilities or visual impairments, and this is not the only issue associated with this type of packaging (16). Although the concept of unit-dose packaging does not suggest reuse after opening, some patients may attempt to save the remaining medication for future use, even if the packaging does not recommend this. Additionally, disposable containers typically do not provide precise dosing of medication drops (73).
Prospects for using eye drops blisters
Recently, there has been growing public concern about the significant amount of plastic waste generated from the use of BFS technology (16). The pharmaceutical sector is responsible for almost 55% more toxic carbon emissions than the automotive industry, and plastic packaging solutions can be considered one of the main contributors to this problem. The manufacturing process of such packaging requires a large number of materials, which creates an additional burden on the environment, both in terms of production and recycling (34). Although attempts are being made to modify the materials used in BFS technology, such as the development of thermoplastic elastomer, this does not necessarily reduce the amount of waste generated from this type of packaging.
Blister packaging may be a more sustainable alternative to BFS, as it is widely used in other dosage forms. Blister packaging is a laminate of polyvinyl chloride (PVC) and Al, and it is the most commonly used packaging format in the pharmaceutical industry. In 2021, the demand for blister packaging reached 5.18 thousand tons (74). Blister packaging has been used as a package for the Orochol® oral cholera vaccine (Berna Biotech Ltd., Bern, Switzerland), which is a two-chamber Al foil product (75). For eye drops, there is a DosePan® single-chamber blister (Pharmapan AG, Möhlin, Switzerland). To create this packaging, Al-based composite materials are used, and the full cycle of blister production consists of 6 technological stages: cutting, cold forming, spout sealing, filling and fragile sealing, permanent sealing, and final cutting (Figure 2).
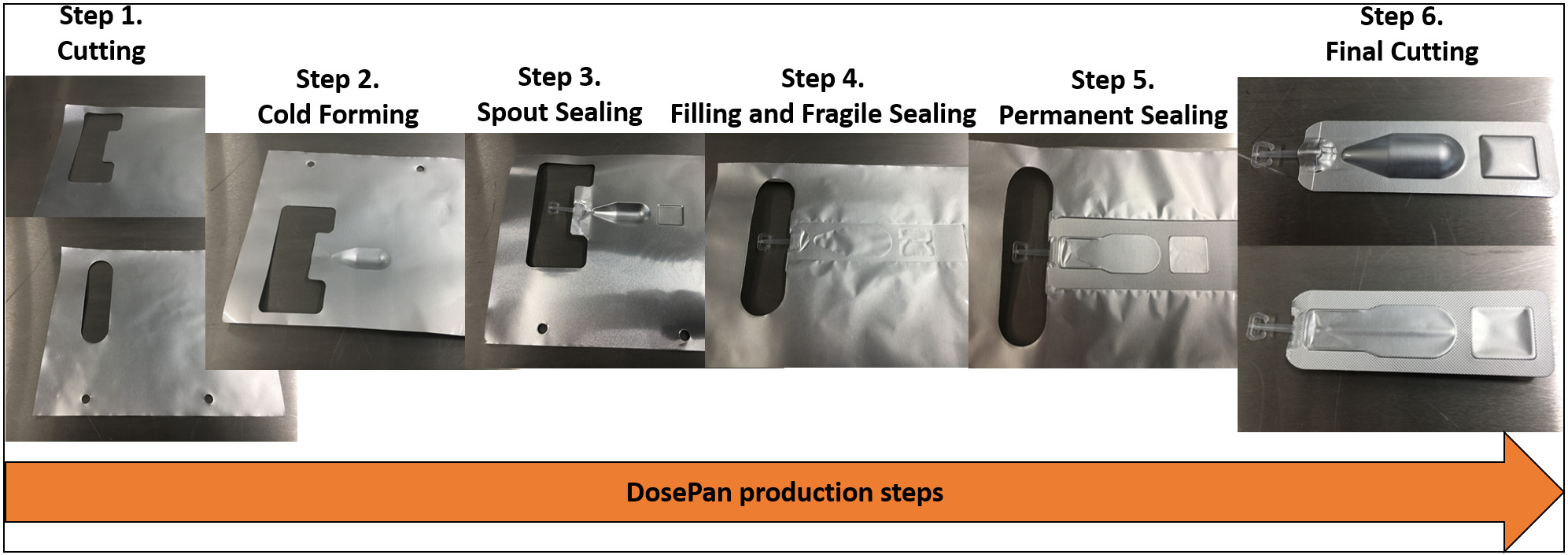
Figure 2. DosePan® production steps.
Compared to BFS, this technology does not require high pressure, and the source material does not need to undergo conversion. Moreover, the material forming stage involves pulling the composite with a punch without using heat. The sealing process takes place only along the perimeter of the package, without affecting the area filled with the preparation. In DosePan®, a brittle seal occurs between the container with the drug and the spout, similar to a two-chamber blister, but the seal collapses when the patient applies force to the container from the base to the spout, unblocking the flow of liquid out.
The material used has a multilayer structure, which enhances the package's performance concerning light and gas permeability. Al is commonly used to ensure proper barrier properties, increasing the structure's rigidity and reducing the size and cost of raw materials for production (69). It also helps prevent the diffusion of contaminants into the formulation and is considered a "green metal" ideal for recycling (76, 77). Other materials like polyethylene contribute sealing properties, reduce the product's cost, and serve as a separating layer that exhibits antistatic properties and prevents charge accumulation. Oriented polyamide, on the other hand, provides better barrier, mechanical, and optical properties, while polyurethane improves layer adhesion (78). Different approaches are used to produce composite materials (79). However, heterogeneous layers make the resulting product challenging to process, as each layer requires different degrees of processing during recycling (80-82). Processing multilayer structures in a single flow is not recommended, as the resulting product will be difficult to use as a secondary raw material (83).
The EU has expressed significant concerns about the recycling of packaging based on flexible materials. In March 2020, the Circular Economy Action Plan was presented, outlining that all packaging in the EU market must be economically viable for reuse or recyclable by 2030 (84). The Circular Economy for Flexible Packaging is an initiative working towards enhancing the versatility of flexible packaging, with specific recommendations developed for its recycling (83). Blister packaging based on PVC and Al is processed through fine grinding, followed by mechanical separation of laminated materials (85). However, the resulting Al may contain up to 10% residual PVC, rendering it unsuitable as a secondary raw material, and with lower quality than primary Al. A more technologically advanced approach is the CreaSolv® process (Fraunhofer Institute IVV, Freising, Germany), which uses selectively active solvents to eliminate the polymer. The CreaSolv® technology aims to produce high-purity secondary Al and a separate polymer fraction (86). The dissolution step in polymer recycling assumes that the targeted polymer will selectively dissolve in the solvent (87). In subsequent cleaning stages, mechanical methods are used to remove components that haven't dissolved. After purification, a solution of macromolecules of the targeted polymer is obtained, which is then separated from the solvent during the precipitation stage. The recycled polymer is dried and the solvent is recovered and reused in the process. Finally, the purified polymer is ready for reuse (88). The NewCycling process (APK AG, Merseburg, Germany), focuses on the separation of polymers in multilayer films containing Al foil by stepwise dissolution of polyethylene and polypropylene in methylcyclohexane with increasing temperature. Saperatec (Saperatec GmbH, Dessau-Rosslau, Germany) and PVC Separation (PVC Separation Pty Ltd, Tonsley, Australia) have developed a special category of separation processes for multilayer films and laminates that use solvents but do not require complete dissolution of the polymers. Saperatec proposes to reduce the interfacial forces between polyethylene terephthalate, polyethylene, and Al foil in multilayer films. According to the patent, this proposed technology uses an emulsion based on an organic solvent for swelling and a carboxylic acid to accelerate separation. The process of delamination of multilayer materials proceeds with swelling of the polymer in a low-boiling solvent. Exposure to hot water causes the solvent to evaporate and the polymers to be released, which are then separated on a sieve due to differences in density (89).
In a previous study, we examined registered eye drops across several countries and observed a notable preference for single-dose medications in the EU (90). Likewise, blister technologies can be effectively applied to the packaging of ophthalmic drugs. Since blister packaging is already popular, this approach can help reduce waste that differs in its qualitative composition. This concept can compete with ophthalmic packaging standards such as BFS and Droptainer® while maintaining equal performance.
Conclusion
The prioritization of preservative-free eye drops stems from their role in preventing additional adverse events among patients. However, this approach introduces a potential risk of contamination in the composition by pathogenic microflora, leading to its entry into the visual organ. Consequently, ensuring sterility in the production process is imperative, primarily achieved through effective primary packaging. The tragic incident in Birmingham during the 1950s underscored the challenges associated with using bottles with pipettes. It can be argued that this event marked the initiation of the modernization of primary packaging for eye drops, a chronology detailed in this article. While the plastic Droptainer® boasts a simple design, it falls short in maintaining sterility. Systems like Comod®, 3K®, ABAK®, Novelia®, and the OSD exhibit a high likelihood of preserving sterility. However, there still exists a risk of contamination in Comod® and ABAK® multidose packaging, exemplified by incidents involving Pseudomonas aeruginosa. In contrast, Novelia® demonstrated a prolonged absence of signs of bacterial contamination and superior control during instillation. The FDA-approved OSD also stands out for its significantly smaller carbon footprint compared to unit-dose equivalents. While unit-dose systems offer the advantage of maintaining drug sterility until use and provide design flexibility, the economic feasibility of such packaging remains a subject of intense debate. Conflicting reports exist regarding the lower production cost of BFS in comparison to progressive multidose packaging. Additionally, questions persist about the recyclability of such packaging and its potential environmental impact. In conclusion, an alternative packaging solution in the form of the DosePan® blister is proposed. This option maintains sterility, offers convenience and safety, and shows promising prospects for recycling. The article presents various existing technologies for recycling flexible packaging, including the renewal of the underlying Al material. The reduction of packaging variety is advocated as a means to alleviate the environmental burden, with the suggestion of combining DosePan® blisters with those used in solid dosage forms for efficient waste recycling.
Declarations
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicts of interest that are directly relevant to the content of this review.
References
- Scope e-Knowledge Center Pvt Ltd. Preservative-Free Ophthalmic Products [Internet]. 2013. Available from: https://iphco.com/Editor/UploadFiles/pdf/paper_4.pdf
- Jumelle C, Gholizadeh S, Annabi N, Dana R. Advances and limitations of drug delivery systems formulated as eye drops. J Control Release. 2020; 321:1–22.
- Akhter MH, Ahmad I, Alshahrani MY, Al-Harbi AI, Khalilullah H, Afzal O, и др. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels. 2022;8(2):82.
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–26.
- Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763–8.
- Templeton WC, Eiferman RA, Snyder JW, Melo JC, Raff MJ. Serratia keratitis transmitted by contaminated eyedroppers. Am J Ophthalmol. 1982;93(6):723–6.
- McCulloch J. Origin and pathogenicity of Ppyocyanea in conjunctival sac. Arch Ophthalmol. 1949;29:924-36.
- Hogan MJ. The preparation and sterilization of ophthalmic solutions. Calif Med. 1949;71(6):414–6.
- Theodore FH. Contamination of Eye Solutions. American Journal of Ophthalmology. 1951;34(12):1764.
- Theodore FH, Feinstein RR. Practical Suggestions for the Preparation and Maintenance of Sterile Ophthalmic Solutions*. American Journal of Ophthalmology. 1952;35(5):656–9.
- Vaughn DG. The Contamination of Fluorescein Solutions With Special Reference to Pseudomonas Aeruginosa (Bacillus Pyocyaneus). American Journal of Ophthalmology. 1955;39(1):55–61.
- Rahman MQ, Tejwani D, Wilson JA, Butcher I, Ramaesh K. Microbial contamination of preservative free eye drops in multiple application containers. British Journal of Ophthalmology. 2006;90(2):139–41.
- Bakhrushina EO, Anurova MN, Demina NB, Lapik IV, Turaeva AR, Krasnuk II. Ophthalmic Drug Delivery Systems (Review). Razrabotka i registraciâ lekarstvennyh sredstv. 2021;10(1):57–66.
- Wilson LA. To preserve or not to preserve, is that the question? British Journal of Ophthalmology. 1996;80(7):583–4.
- Kim MS, Choi CY, Kim JM, Chung HR, Woo HY. Microbial contamination of multiply used preservative-free artificial tears packed in reclosable containers. British Journal of Ophthalmology. 2008;92(11):1518–21.
- Birkhoff M, Marx D. Ophthalmic squeeze dispenser - Eliminating the Need for Additives in Multidose Preservative-Free Eyecare Formulations [Internet]. Drug Development and Delivery. 2017 [cited on December 21, 2023]. Available from: https://drug-dev.com/ophthalmic-squeeze-dispenser-eliminating-the-need-for-additives-in-multidose-preservative-free-eyecare-formulations/
- Erichev VP, Petrov SYu, Volzhanin AV, Ghazaryan SA. Continuous anti-glaucoma drug therapy as a risk factor of dry eye. Vestn oftal’mol. 2019;135(6):117.
- Campolo A, Crary M, Shannon P. A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops. BJSTR [Internet]. July 5, 2022 [cited on 21 December 2023];45(1). Available from: https://biomedres.us/fulltexts/BJSTR.MS.ID.007130.php
- Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular Symptoms and Signs with Preserved and Preservative-Free Glaucoma Medications. European Journal of Ophthalmology. 2007;17(3):341–9.
- Hsu KH, Gupta K, Nayaka H, Donthi A, Kaul S, Chauhan A. Multidose Preservative Free Eyedrops by Selective Removal of Benzalkonium Chloride from Ocular Formulations. Pharm Res. 2017;34(12):2862–72.
- Petrov S. Tafluprost--a novel prostaglandin F2alpha analogue. Vestn Oftalmol. 2014;130(5):85–95.
- Bouwman-Boer Y, Fenton-May V, Le Brun P. Practical Pharmaceutics An International Guideline for the Preparation, Care and Use of Medicinal Products [Internet]. 5-е изд. New York: Springer Cham; 2015. 878 с. Available from: https://link.springer.com/book/10.1007/978-3-319-15814-3
- Bohrer D, Do Nascimento PC, Binotto R, Carlesso R. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part II: Amino acids for parenteral nutrition. Journal of Trace Elements in Medicine and Biology. 2001;15(2–3):103–8.
- Ogawa T, Miyajima M, Wakiyama N, Terada K. Effects of Phosphate Buffer in Parenteral Drugs on Particle Formation from Glass Vials. Chem Pharm Bull. 2013;61(5):539–45.
- Guadagnino E, Guglielmi M, Nicoletti F. Glass: The best material for pharmaceutical packaging. International Journal of Applied Glass Science. 2022;13.
- Sumitra A, Dhawal C, Dileep U, Nagasuri R. Pharmaceutical Glass Interactions: A Review of Possibilities. J Pharm Sci & Res. 2016;8(2):103–11.
- Pareek V, Khunteta A. Pharmaceutical packaging: current trends and future. 2014;6(6):480–5.
- Joseph T, Hasanin M, Unni A, Kar Mahapatra D, Haponiuk J, Thomas S. Macromolecules: Contemporary Futurist Thoughts on Progressive Journey. Eng. 2023;4(1):678–702.
- King JH. Contamination of Eye Medications: Practical Methods of Prevention. American Journal of Ophthalmology. 1953;36(10):1389–97.
- Research C for DE and. Container Closure Systems for Packaging Human Drugs and Biologics [Интернет]. FDA; 2020 [cited on December 21, 2023]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/container-closure-systems-packaging-human-drugs-and-biologics
- Al-Jumaian N, Malik R, Khandekar R, Al-Humaidan A, Al-Madany R, Al-Qahtani R, и др. Bottle characteristics of topical international glaucoma medications versus local brands in Saudi Arabia. Middle East Afr J Ophthalmol. 2016;23(4):296.
- Moore D, Hammer J, Akhtari R, Beck J, Sanders S, Kryscio R. Squeeze Me if You Can: Variability in Force Requirements to Extract a Drop From Common Glaucoma Bottles. Journal of Glaucoma. 2016;25(9):780–4.
- Karki R, Meena M, Prakash T, Rajeswari T, Goli D, Kumar S. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J Adv Pharm Tech Res. 2011;2(3):192.
- Birkhoff M. Delivering on the Growing Need for Topical Preservative-Free Ophthalmic Treatments. ONdrugDelivery Magazine. 2020;(104):8–12.
- Birkhoff M, Marx D. New devices for dispensing ophthalmic treatments may be the key to managing the life cycles of established products. 2010;10:16–21.
- Prasher P, Singh M, Mudila H. Oligodynamic Effect of Silver Nanoparticles: a Review. BioNanoSci. 2018;8(4):951–62.
- Teping C, Wiedemann B. Das COMOD®-System - Ein konservierungsmittelfreies Mehrdosenbehältnis für Augentropfen. Klin Monatsbl Augenheilkd.1994;205(10):210–7.
- Drew T, Wolffsohn J. Usability of prostaglandin monotherapy eye droppers. Br J Ophthalmol. 2015;99(9):1251–4.
- Laboratoires Théa. ABAK. Pure technology in a bottle. 2011; Available from: https://thea.pt/sites/default/files/documentos/abak_-_pure_technology_in_a_bottle.pdf
- Gabisson P, Briat B, Le Foll J, Conan S, Bale-Le Bescond F, Talmud M. Maniabilité et acceptabilité du flacon Abak nouvelle génération chez des patients traités au long cours. Étude transversale, rétrospective et multicentrique. Ann Pharm Fr. 2011;69(1):22–9.
- Kashiwagi K. Wide Variation of Squeezing Force and Dispensing Time Interval among Eyedropper Bottles. Journal of Ophthalmology. 2019;2019:e7250563.
- Malet F, Karsenti D, Pouliquen P. Preservative-Free Ocular Hydrating Agents In Symptomatic Contact Lens Wearers: Saline versus PVP Solution: Eye & Contact Lens: Science & Clinical Practice. 2003;29(1):38–43.
- Guillon M, Maissa C, Pouliquen P, Delval L. Effect of Povidone 2% Preservative-free Eyedrops on Contact Lens Wearers With Computer Visual Syndrome:: Pilot Study. Eye & Contact Lens: Science & Clinical Practice. 2004;30(1):34–9.
- Da Costa A, Yu MC, De Freitas D, Cristovam P, LaMonica L, Dos Santos V, et al. Microbial Cross-contamination in Multidose Eyedrops: The Impact of Instillation Angle and Bottle Geometry. Trans Vis Sci Tech. 2020;9(7):7.
- Ozulken K, Cubuk M, INAN N, Acar U, Gocmen J, Akman A. The comparison of bacterial contamination and antibacterial effi cacy of the anti-glaucomatous eyedrops with and without preservatives. Journal of Glaucoma and Cataract. 2020;15:118.
- Kaarniranta K, Ropo A. Preferences and ease of use of preservative-free IOP-lowering eye drop containers: A comparison of two multi-dose bottles. Clinical-Investigation [Internet]. 2018 [cited on December 21, 2023];07(04). Available from: http://www.openaccessjournals.com/articles/preferences-and-ease-of-use-of-preservativefree-ioplowering-eye-drop-containers-a-comparison-of-two-multidose-bottles-12319.html
- Roche M, Lannoy D, Bourdon F, Danel C, Labalette P, Berneron C, и др. Stability of frozen 1% voriconazole eye-drops in both glass and innovative containers. European Journal of Pharmaceutical Sciences. 2020;141:105102.
- Strauss RA, Genschel U, Allbaugh RA, Sebbag L, Ben‐Shlomo G. Evaluation of microbial contamination of canine plasma eyedropper bottles following clinical use in canine patients. Veterinary Ophthalmology. 2019;22(3):222–8.
- Sellier F. User testing: critical for truly understanding patient needs. 2019;(94):36–8.
- Murray M, Shojanja K. Unit-dose drug distribution systems. Am J Health Syst Pharm. 2000;(56):101–9.
- Buchanan C. A Brief History of Unit-Dose Drug Distribution. J Pharm Technol. 1(3):127–9.
- Scherer R. Collapsible dispensing capsule. US 2134489, 1938.
- Swarbrick J, Boylan J. Encyclopedia of Pharmaceutical Technology. Т. 20. New York: Marcel Dekker, Inc.; 2009. 335 p.
- Oschmann R, Schubert OE. Blow-Fill-Seal Technology (Paperback Apv Band 40). 1st edition. Medpharm; 132 p.
- Gerhard H. Improvements relating to blow moulding machines. GB 1041548A, 1966.
- Ljungqvist B, Reinmüller B, Löfgren A, Dewhurst E. Current Practice in the Operation and Validation of Aseptic Blow-Fill-Seal Processes. PDA Journal of Pharmaceutical Science and Technology. 2006;60(4):254–8.
- Haerer M, Muhvich K, Akerman P. The Manufacture of Sterile Pharmaceutical Products Using Blow-Fill-Seal Technology. Parenteral Drug Association, Inc.; 2017. Report No.: 77.
- Leon A. Improving Sterility Using Blow-Fill-Seal Technology. Pharmaceutical Technology. 2022;46(10):37, 51–37, 51.
- Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: The good, the bad and the ugly. Progress in Retinal and Eye Research. 2010;29(4):312–34.
- Hertel F, Pfeiffer N. Einzeldosisapplikationen in der Glaukomtherapie. Vergleich der Kosten mit Mehrdosis. Ophthalmologie. 1994;(91):602–5.
- Herbig E. Monitoring Airborne Micro-organisms in Blow-Fill-Seal Technology. Pharmaceutical Technology Europe [Internet]. June 1, 2004 [cited on December 21, 2023];16(6). Available from: https://www.pharmtech.com/view/monitoring-airborne-micro-organisms-blow-fill-seal-technology
- Ljungqvist B, Reinmuller B. Cleanroom design: minimizing contamination through proper design. 1st edition. Boca Raton: CRC Press; 1997. 156 p.
- Poisson P. Non-Viable Particle Management During B/F/S Manufacturing Operations. (Autumn Edition):12–6.
- Poisson P, Reed C, Sinclair C. Challenge Testing of the KleenKut Parison Cut off Mechanism. BFS User’s Group Annual Meeting; 2001 June 14; Switzerland.
- Bradley A, Probert SP, Sinclair CS, Tallentire A. Airborne microbial challenges of Blow/Fill/Seal equipment: a case study. J Parenter Sci Technol. 1991;45(4):187–92.
- Sinclair CS, Tallentire A. Performance of blow/fill/seal equipment under controlled airborne microbial challenges. PDA J Pharm Sci Technol. 1995;49(6):294–9.
- Sundström S, Ljungqvist B, Reinmüller B. Some observations on airborne particles in blow-fill-seal filling rooms. PDA J Pharm Sci Technol. 2007;61(3):147–53.
- Piringer O, Baner A. Plastic Packaging: Interactions with Food and Pharmaceuticals, 2nd, Completely Revised Edition. Germany: Weinheim: Wiley; 2008. 632 p.
- Hroncich C. Examining Blow-Fill-Seal Technology for Aseptic Processes. Pharmaceutical Technology. 2016;40(6):49–51.
- Markarian J. Considering Blow-Fill-Seal for Biologic Drugs. Pharmaceutical Technology. 2022;46(4):33-34,57.
- Amin A, Dare M, Sangamwar A, Bansal AK. Interaction of antimicrobial preservatives with blow-fill-seal packs: correlating sorption with solubility parameters. Pharmaceutical Development and Technology. 2012;17(5):614–24.
- Hapa AG. For the first time, a CMYK digital printing process for BFS products [Internet]. 2020 [cited on April 14, 2023]. Available from: https://www.hapa.ch/en/news/for-the-first-time-a-cmyk-digital-printing-process-for-bfs-products
- Bagnis A, Papadia M, Scotto R, Traverso CE. Antiglaucoma drugs: The role of preservative-free formulations. Saudi Journal of Ophthalmology. 2011;25(4):389–94.
- Dalal S, Dalal P, Motiani R, Solanki V. Experimental Investigation on Recycling of Waste Pharmaceutical Blister Powder as partial replacement of Fine aggregate in Concrete. Resources, Conservation & Recycling Advances. 2022;14:200076.
- Papania M, Zehrung D, Jarrahian C. Technologies to Improve Immunization. В 2018. p. 1320-1353.
- Marsh K, Bugusu B. Food Packaging—Roles, Materials, and Environmental Issues. Journal of Food Science [Internet]. April 2007 [cited on December 21, 2023];72(3). Available from: https://ift.onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2007.00301.x
- Fedorov P, Samoylov A. Ancient Roman technology of aluminum production: Process reconstruction. Fine Chemical Technologies. 2020;14:31–8.
- Sudoł E, Kozikowska E. Mechanical Properties of Polyurethane Adhesive Bonds in a Mineral Wool-Based External Thermal Insulation Composite System for Timber Frame Buildings. Materials. 2021;14(10):2527.
- Azevedo AG, Barros C, Miranda S, Machado AV, Castro O, Silva B, и др. Active Flexible Films for Food Packaging: A Review. Polymers. 2022;14(12):2442.
- Tacker M, Pauer E, Krauter V. Sustainability of flexible multilayer packaging: Environmental impacts and recyclability of packaging for bacon in block. Cleaner Environmental Systems. 2020;1.
- Mumladze T, Yousef S, Tatariants M, Kriūkienė R, Makarevicius V, Lukošiūtė SI, и др. Sustainable approach to recycling of multilayer flexible packaging using switchable hydrophilicity solvents. Green Chem. 2018;20(15):3604–18.
- Coltelli M, Gigante V, Cinelli P, Lazzeri A. Flexible Food Packaging Using Polymers from Biomass. В 2019. p. 272–96.
- Dorijan N. Recycling as a challenge for the flexible packaging industry. Journal of Economic and Social Development. 8(2):2–8.
- Circular Economy Action Plan. For a cleaner and more competitive Europe [Internet]. European Commission; 2020 [cited on April 21, 2023]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN
- Hagelüken C, Lee-Shin J, Carpentier A, Heron C. The EU Circular Economy and Its Relevance to Metal Recycling. В: Recycling [Internet]. 2016 [cited on December 21, 2023]. p. 242–53. Available from: http://www.mdpi.com/2313-4321/1/2/242
- Knappich F, Hartl F, Schlummer M, Mäurer A. Complete Recycling of Composite Material Comprising Polybutylene Terephthalate and Copper. Recycling. 2017;2:9.
- Schlummer M, Wolff F, Maurer A. Recovery of PC/ABS from WEEE plastic shred by the CreaSolv® process. В 2016. p. 1–6.
- Strobl L, Diefenhardt T, Schlummer M, Bielmeier T, Wagner S. Recycling Potential for Non-Valorized Plastic Fractions from Electrical and Electronic Waste. Recycling. 2021;6:33.
- Vollmer I, Jenks M, Roelands MCP, White RJ, Harmelen T van, Wild P de, и др. Beyond Mechanical Recycling: Giving New Life to Plastic Waste [Internet]. 2020 [cited on December 21, 2023]. Available from: https://www.researchgate.net/publication/339852672_Beyond_Mechanical_Recycling_Giving_New_Life_to_Plastic_Waste
- Ivanov IS, Bakhrushina EO, Turaeva AR, Shatalov DO, Aydakova AV, Akhmedova DA, et al. Approaches to the search of the optimum packaging of eye drops. International Journal of Applied Pharmaceutics. 2022;1–7.

 ETFLIN
Notification
ETFLIN
Notification







