Antidiabetic and Antihyperlipidemic Activity of Ethanolic Extract of Mentha viridis in Alloxan Induced Diabetic Rats
by Nusrat Jahan Juthy, Gazi Jahirul Islam , Abdullah Zehad, Shaheda Zannah ★
Academic editor: Pilli Govindaiah
Sciences of Pharmacy 3(3): 167-176 (2024); https://doi.org/10.58920/sciphar0303258
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
22 Jun 2024
12 Sep 2024
18 Sep 2024
30 Sep 2024
Abstract: This research was designed to examine the phytochemicals of Mentha viridis (M. viridis) ethanolic extract and the antidiabetic and antihyperlipidemic activities in alloxan-induced animal models. Diabetes was induced chemically by administering a unit dose of alloxan at 120 mg/kg BW. After alloxan induction, hyperglycemic rats were dealt with ethanolic extract of leaf and whole plant, metformin, and a mixture of leaf extract with metformin and whole plant extract with metformin for two weeks. Ethanolic extract of leaf and whole plant, metformin, and a combination of both leaf and whole plant extract with metformin therapies reduced glucose levels in the blood compared with the diabetic negative control group after two weeks of treatment. However, among the therapies, the ethanolic leaf extract and the combination of whole plant extracts with metformin were found to be the most effective (p<0.05), with reductions of 62.82% and 72.89%, respectively. After diabetes induction, the serum level of TG (triglycerides), TC (total cholesterol), LDL-C (low-density lipoprotein-cholesterol) escalated notably (p<0.05), and HDL-C (high-density lipoprotein-cholesterol) level decreased remarkably (p<0.05) in hyperglycemic rats as opposed to healthy normal rats. Ethanolic leaf extract and a combination of whole plant extract with metformin significantly minimized the elevated extent of TG and LDL-C. They surged HDL-C, but the TC level was reduced by whole plant extract only after two weeks of treatment. The standard procedures were used to identify the phytochemical compounds of the medicinal plant M. viridis. The phytochemical compounds such as alkaloids, resins, tannins, phenols, flavonoids, steroids, and terpenoids appeared in the ethanolic leaf extract of M. viridis. The findings suggest that M. viridis might provide better glycemic control and hypolipidemic effect in diabetic rats when administered alone or combined with oral antidiabetic agents. Incorporating M. viridis extract with metformin in improving hyperglycemic and hyperlipidemic conditions in diabetic rats proves that M. viridis has a synergistic effect, which could enhance the antidiabetic activity of oral hypoglycemic agents.
Keywords: Metformin combinationHerbal synergistic effectOral hypoglycemic agent
Introduction
Diabetes mellitus refers to a group of heterogeneous metabolic disorders primarily characterized by chronic hyperglycemia, defined as a persistent elevation in blood glucose levels. The primary cause is either a disruption in insulin secretion, varying degrees of insulin resistance, or, more commonly, a combination of both. Diabetes mellitus, if left untreated, inadequately treated, or undiagnosed over a longer period, is most often correlated with the increased risk of cardiovascular diseases, kidney disorders, blindness, and foot cutoff (1, 2). According to IDF (International Diabetes Atlas, 10th edition), in the 21st century, diabetes is the fastest-growing health emergency worldwide. It has been projected that about 643 million people will have diabetes by 2030 and 783 million by 2045 (3). Diabetes mellitus substantially affects food nutrients such as carbohydrate, fat, and protein metabolism and sequentially causes chronic hyperglycemia followed by lipid profile abnormalities. Long-term untreated or poorly treated hyperglycemia considerably induces numerous microvascular and macrovascular diabetic complexities that are the ultimate reasons for diabetes-related morbidity and mortality (4).
The accessible medications for diabetes are insulin and varied oral antidiabetic agents like biguanides, sulfonylureas, thiazolidinediones, non-sulfonylureas secretagogues, and α-glucosidase inhibitors, etc., alongside insulin (5, 6). Therefore, these drugs are used as monotherapy or combined to get better glycemic control and mask serious adverse effects of each oral antidiabetic agent (7). Over the last few decades, numerous studies have been done to find any potential in medicinal plants alone or a combination of oral antidiabetic agents in improving hyperglycemia and associated complications of DM in animal models (8). Plenty of plants have been carefully evaluated as a primary origin of dominant antidiabetic agents because herbal plants are a rich wellspring of phytoconstituents with insignificant toxicity or no side effects, making them a potential therapeutic choice for treating diabetes (9-11). Moreover, herbal medicine offers treatment at a cheaper rate than conventional medicine (9). An extensive review by Salehi et al. has covered many medicinal plants claimed to possess antidiabetic activity (8).
Mentha viridis (M. viridis), also known as Mentha spicata or spearmint, is a medicinal plant member of the Lamiaceae family widely grown in Europe, Asia, and North America but currently cultivated worldwide (12). This medicinal plant has many beneficial effects in its phytoconstituents and is utilized in numerous disorders such as diabetes, respiratory diseases, and skin disorders (13-15). According to Benkhnigue et al., for diabetes therapy, the leaf of M. viridis is orally given as a decoction in the locality of Al Haouz-Rhamna in Morocco (16). In another study reported by Idm’ hand et al., the leaf and stem of M.viridis are used to treat diabetes as a decoction or infusion form (13). Aqueous ethanolic extract of M. viridis exhibited blood glucose lowering and hypolipidemic effect in alloxan-caused diabetic rats (17). Additionally, aqueous leaf extract showed positive results in hyperglycemia and lipid abnormalities in diabetic animals (18). Phenolic leaf extract of M. viridis had antidiabetic effects in chemically induced diabetic rats, as reported elsewhere (19).
Notably, numerous studies reported that preliminary screening of M. viridis disclosed the existence of phytoconstituents, for example, tannins, polyphenols, steroids, flavonoids, triterpenes, and glycosides (20). Per one study, ethanolic extracts of M. viridis contain a substantial amount of phenolic compounds, including polyphenols, flavonoids, and caffeic acid derivatives (21). In essential oils extracted from M. viridis, carvone was an entire primary component besides trans-carveol, limonene, linalool, menthone, piperitone, piperitone oxide, and isomenthone (8). This research work was aimed to investigate the chemical composition of the ethanolic extract of M. viridis and to evaluate the antihyperglycemic and antihyperlipidemic activities of M. viridis separately and in blending with metformin in chemically induced diabetic rats by alloxan.
Methods and Materials
Chemicals
All the chemicals employed in this investigation were of analytical grade. Alloxan was bought from German-based Sigma Chemicals. Glucose was procured from Glaxo Smith Kline. The rest of the chemicals, like triglycerides (TG), ligh-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) kits, were obtained from (LINEAR CHEMICALS S.L., Spain). The reference drug, metformin, was purchased from Chadwell Health Essex, England. The required solutions were prepared on each day of the experiment.
Plant Collection
Leaves and barks of M. viridis were collected from Kawranbazar, Dhaka 1215, Bangladesh. The whole plant (leaves and barks) was identified by a Bangladesh National Herbarium specialist in Mirpur, Dhaka, Bangladesh. The accession number is DACB–41939.
Extract Preparation
Leaves and barks of M. viridis were rigorously cleaned with water and afterwards dried for seven days under sunlight. The coarse powder was obtained from the plant parts after grinding with an appropriate grinder machine. The dried and powdered materials (100 g) from each plant part were immersed in 500 mL of 90% ethanol for two weeks at ambient temperature with periodic shaking. Initially, a cotton filter and finally, a Whatman No. 1 filter paper were utilized to filter the solution. A rotary evaporator from Bibby Sterlin Ltd, UK, concentrated the filtrate at 40°C. A semisolid extract (2.08 g each) was obtained when the extra solvent evaporated.
Experimental Animal
Long Evans male rats (100–120 g) aged nine weeks were obtained from the ICDDR, B (International Centre for Diarrheal Disease Research, Bangladesh). The standard atmospheric states, such as 22–25°C temperature, 60–65% humidity, light/dark cycle (12/12 h), etc., were maintained when rats were kept in animal cases. Throughout the experiment, all rats were given food like standard laboratory diet (Purina rat chow) from ICDDR, B, Dhaka, and pure drinking water. All animal experiments were performed after the agreement with the Committee of Animal Ethics of Southeast University, Department of Pharmacy.
Acute Toxicity Studies
Long Evans male rats were fasted overnight and were selected for the study. Each extract (whole plant and leaf extract only) was administered orally to two groups (n = 5) of rats. The doses were 250 mg/kgBW and 500 mg/kgBW (for whole plant and leaf extract). Following administration of all the extracts and metformin, the animals were perceived intimately, particularly for the initial three h, for expression in abnormalities, such as salivation, surged motor activity, chronic convulsions, coma, and death. Regular inspections were done at a uniform gap for a single whole day. This regular monitoring was continued for 4 days.
Organizing of Investigational Rats
Long-Evans rats were arbitrarily allocated into 7 groups. Each group contains five rats (n = 5) and utilized test studies, including the blood glucose estimation, evaluation of lipid profile, etc., following 14-day treatment protocols.
- Group I: Healthy normal rats with no treatment (Normal control)
- Group II: Diabetic control rats (Untreated Group) (Negative control)
- Group III: Diabetic rats administered leaf extract (500 mg/kgBW)
- Group IV: Diabetic rats administered herb extract (500 mg/kgBW)
- Group V: Diabetic rats administered metformin (850 mg/70 kgBW)
- Group VI: Diabetic rats administered a blending of leaf extract (250 mg/kgBW) and metformin (425 mg/70 kgBW)
- Group VII: Diabetic rats administered a blending of whole plant extract (250 mg/kgBW) and metformin (425 mg/70 kgBW)
Diabetes Induction
A newly prepared alloxan solution (120 mg/kgBW) in distilled water was injected intraperitoneally singly into each rat after 12 h of overnight fasting. These animals were given a 10% glucose solution to drink to deal with alloxan-induced low blood sugar, as there was an instantaneous rise in blood insulin just after the alloxan injection within minutes, called the initial transient hypoglycemic phase (22, 23). Blood glucose content was estimated from the tail vein of diabetic rats 72 h later. With marked hyperglycemia (FBG (fasting blood glucose) ≥25.70 mmol/L were chosen for the successive investigation.
Preparation of Dosage of Reference Drug and Plant Extract
Preparation
of Extract Solution (Leaf and Whole Plant)
The extracts (leaf and whole plants) were semisolid and sparingly soluble in water. The suspension form of the water dosages was prepared so that each 0.1 mL of solution contained plant extract as specified by the 500 mg/kgBW dose.
Preparation
of Metformin Solution
The physical appearance of metformin was a white crystalline solid and was highly soluble in water. That’s why the dosages were prepared in solution form using distilled water so that each 0.1 mL of solution contained metformin following the dose of 850 mg/70 kgBW. In humans, this drug works effectively in the same dose.
Preparation of Leaf Extract and Metformin Combination
The dosage was prepared individually so that each 0.1 mL of solution contained leaf extract and metformin in line with the dose of 250 mg/kgBW of leaf extract and 425 mg/70 kgBW of metformin in the given order.
Preparation of Whole Plant Extract and Metformin Combination
The dosage was prepared individually so that each 0.1 mL of solution contained plant extract and metformin as per the dose of 250 mg/kgBW of plant extract and 425 mg/70 kgBW of metformin in the order given.
Blood Serum Collection
Following the completion of the two weeks of treatment with the drug and extracts (whole plant and leaf), chloroform was employed to anesthetize the rats. After confirming that the rats had become unconscious, the thoracic artery was opened by cutting their abdominal skin. 3–4 mL of blood collected directly from the thoracic artery by syringe immediately after opening their skin. The blood sample was centrifuged at 4000 rpm (rotate per minute) for 20 min using a centrifuge machine (Digisystem Laboratory Instrument Inc. Taiwan). The supernatant plasma samples were decanted and stored at -4°C until biochemical examinations were done.
Lipid
Profile
Lipid profile parameters like TC, TG, HDL-C, and LDL-C were estimated colorimetrically by a hematology analyzer using wet reagent diagnostic kits from Randox, UK.
Phytochemical Screening
Ethanolic leaf extract of M. viridis was utilized to carry out the phytochemical screening. Standard procedures were followed to test the phytochemicals such as alkaloids, carbohydrates, flavonoids, resins, saponins, steroids, tannins, and phenols (24–27).
Alkaloids
In a beaker, plant extract of M. viridis (2 mg), distilled water (5 mL), and 1% hydrochloric acid (8 mL) were taken and stirred carefully and very gently until a reaction happened. In 2 mL of this mixture, Dragendorff’s reagent (1 mL) was put on dropwise. The appearance of turbidity or precipitate designates the existence of alkaloids.
Carbohydrates
10 mL of distilled water mixed with 2 mg of plant extract was filtered, and the filtrate was condensed afterwards. Newly prepared 20% α-naphthol (2 drops) was added into this filtrate, and then 2 mL of concentrated sulphuric acid was added dropwise. The formation of a red violet ring indicates the presence of carbohydrates, which fades away from the surplus inclusion of alkali.
Flavonoids
Plant extract (2 mg) was dissolved in ethanol (5 mL) and filtered in a small beaker. Concentrated hydrochloric acid (a few drops) was added carefully to this filtrate. Then, a small piece of magnesium was incorporated, and the pink or reddish coloration proved the presence of flavonoids.
Resins
Plant extract (1 mL) was mixed with copper acetate. The solution was shaken vigorously and left for a few minutes to separate. A green color appearance in the solution demonstrated the presence of resins.
Saponins
Plant extract (0.5 mg) was dissolved in 10 mL of distilled water. Then, the solution was shaken, covered, and left for 30 min. The solution formed a honeycomb-like foam that denoted the appearance of saponins.
Steroids
A 0.2 mg of dry extract was shaken with 2 mL of chloroform, and then concentrated sulphuric acid was added to this mixture carefully by the sides of the test tube. The appearance of a reddish-brown color at the interphase revealed the presence of steroids.
Tannins
10 mL distilled water was mixed with 1–2 mg of plant extract and filtered. A few drops of 0.1% FeCl3 solution were added gently to this filtrate. The presence of tannins was confirmed by the green, blue-green, or blue-black precipitate.
Phenols
Plant extract (0.2 mg) was dissolved in a 5% FeCl3 solution. The formation of green precipitate specified the presence of phenols.
Terpenoids
Plant extract (0.5 mg) was added in 2 mL chloroform, and then the solution was mixed with 3 mL concentrated sulphuric acid. The appearance of a reddish-brown color confirmed the presence of terpenoids.
Statistical Analysis
The statistical analysis was carried out by one-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc test or students paired or unpaired T-test where applicable. The results were represented as mean ± SEM (standard error of the mean). Results were set as significant when p<0.05.
Results
Effect on Blood Glucose
Level
After diabetes induction, group III to group VII rats were treated with the ethanolic extract of leaf and whole plant of M. viridis and a combination of metformin and plant extract (leaf and whole plant) for two weeks. The effects of handling extracts and combination therapy for two weeks on blood glucose levels in alloxan-induced diabetic rats were represented in Figure 1. As can be seen, the BGL (blood glucose level) was decreased significantly (62.82%) (p<0.05) with leaf extract (500 mg/kgBW), and (72.89%) (p<0.05) for the combination of whole plant and metformin (250 and 425 mg/70kgBW), rather than single whole plant extract (43.76%), and combination therapy of metformin with leaf extract (45.88%) compared to untreated DC rats (Group II) (31.80 mmol/L). On the other hand, singly metformin (850 mg/70 kgBW) decreased BGL (65.11%), which was notable (p<0.05) in contrast to the untreated DC rats. The leaf extract decreased blood glucose levels from 31.8 mmol/L to 4.9 mmol/L, which was very significant (p<0.05).
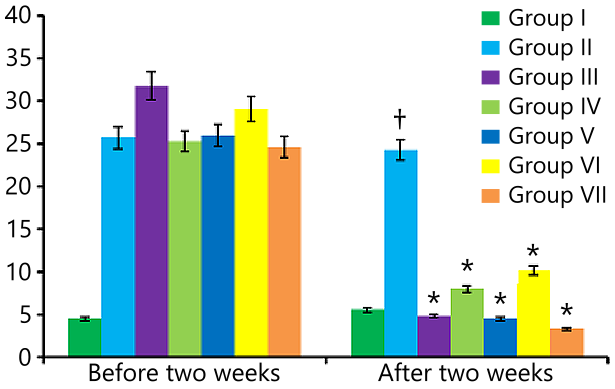
Figure 1. Glucose level of blood in diabetic rats after two weeks oral administration of M. viridis extract, metformin, and combination therapy. All experiments were done in triplicate. Data is presented as mean ± SEM; n = 5 for each group. *p<0.05 contrasted to untreated diabetic control rats. †p<0.05 contrasted to normal rats.
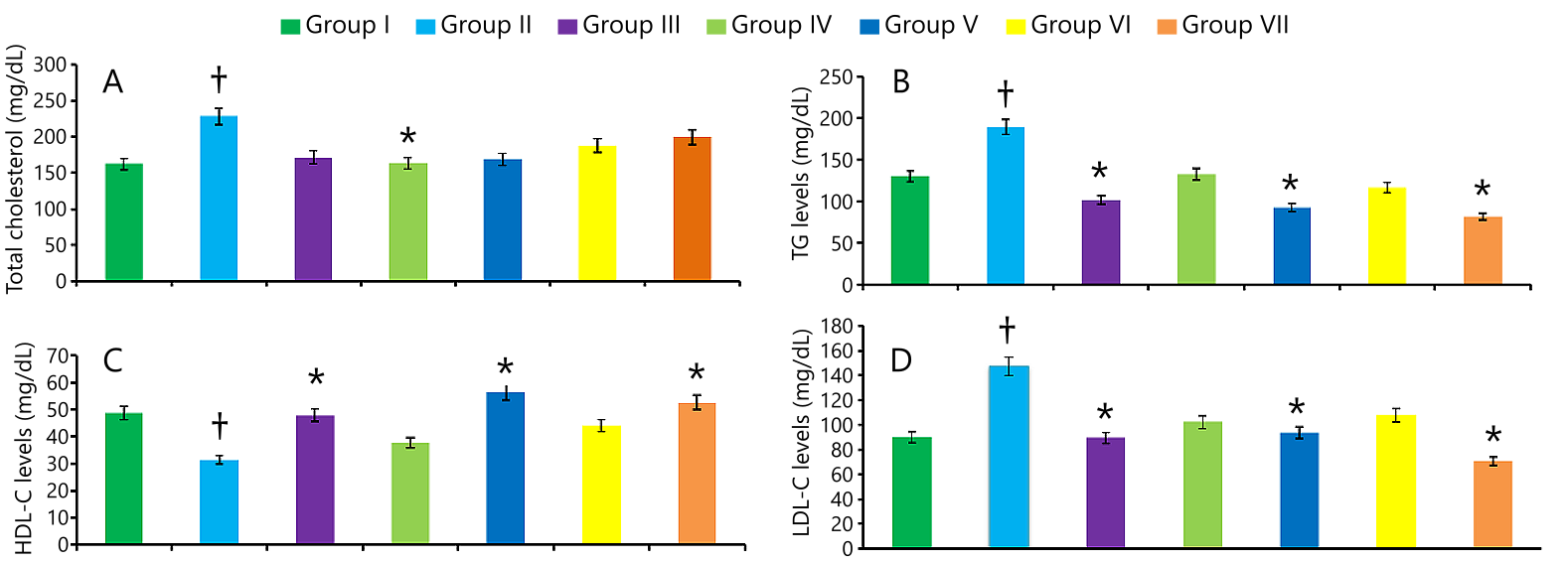
Figure 2. Total cholesterol (A), TG levels (B), HDL-C levels (C), and LDL-C levels (D) in diabetic rats after two weeks oral administration of M. viridis extract, metformin, and mixture of metformin and extracts. All experiments were done in triplicate. Data is presented as mean ± SEM; n = 5 for each group. *p<0.05 contrasted to untreated diabetic control rats. †p<0.05 contrasted to normal rats.
In the other treatment, combining whole plant extract with metformin decreased BGL from 24.6 mmol/L to 3.4 mmol/L compared to the untreated DC group, which was also significant (p<0.05). Notably, all the treatments, leaf extract, whole plant extracts, single metformin, and both the combination therapies reduced the blood glucose level remarkably (p<0.05) in treated diabetic groups (III-VII) as opposed to group II (untreated DC group) (see Figure 1). Here, one combination therapy of metformin with whole plant extract showed the maximum reduction in BGL, which was 72.89%.
Effect on Lipid Profiles
|
Phytochemical constituents |
Result |
|
Alkaloids |
+ |
|
Carbohydrates |
- |
|
Resins |
+ |
|
Terpenoids |
+ |
|
Tannins |
+ |
|
Saponins |
- |
|
Flavonoids |
+ |
|
Phenols |
+ |
|
Steroids |
+ |
Preliminary Phytochemical Screening
Discussion
Conclusion
Declarations
Acknowledgment
All authors are thankful to the Department of Pharmacy, Southeast University for providing all the support and equipment to conduct this research work. Special thanks to ICDDRB for providing experimental rats. Authors also thank the Bangladesh National Herbarium for identifying our experimental plants.
Ethics Statement
All animal experiments were conducted in accordance with the approval of the Animal Ethics Committee of Southeast University, under notification letter number SEU/Pharm/CECR/122/2023.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Petersmann A, Nauck M, Müller-Wieland D, Kerner W, Müller UA, Landgraf R, et al. Definition, classification and diagnostics of diabetes mellitus. Journal of Laboratory Medicine. 2018;42(3):73-9.
- Schleicher E, Gerdes C, Petersmann A, Müller-Wieland D, Müller UA, Freckmann G, et al. Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2022;130(S 01):S1-S8.
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes research and clinical practice. 2022;183:109119.
- Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna journal of medicine. 2020;10(04):174-88.
- de Souza BVC, Moreira Araújo RSdR, Silva OA, Faustino LC, Gonçalves MFB, Dos Santos ML, et al. Bauhinia forficata in the treatment of diabetes mellitus: a patent review. Expert opinion on therapeutic patents. 2018;28(2):129-38.
- Jugran AK, Rawat S, Devkota HP, Bhatt ID, Rawal RS. Diabetes and plant‐derived natural products: From ethnopharmacological approaches to their potential for modern drug discovery and development. Phytotherapy Research. 2021;35(1):223-45.
- Zhou K, Pedersen HK, Dawed AY, Pearson ER. Pharmacogenomics in diabetes mellitus: insights into drug action and drug discovery. Nature Reviews Endocrinology. 2016;12(6):337-46.
- Salehi B, Ata A, V. Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10):551.
- Arumugam G, Manjula P, Paari N. A review: Anti diabetic medicinal plants used for diabetes mellitus. Journal of Acute Disease. 2013;2(3):196-200.
- Singab AN, Youssef FS, Ashour ML. Medicinal plants with potential antidiabetic activity and their assessment. Med Aromat Plants. 2014;3(151):2167-0412.
- Ríos JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta medica. 2015;81(12/13):975-94.
- Liu KH, Zhu Q, Zhang JJ, Xu JF, Wang XC. Chemical composition and biological activities of the essential oil of Mentha spicata Lamiaceae. Advanced Materials Research. 2012;524:2269-72.
- Idm’hand E, Msanda F, Cherifi K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clinical Phytoscience. 2020;6:1-32.
- Jamila F, Mostafa E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. Journal of ethnopharmacology. 2014;154(1):76-87.
- Salhi N, Bouyahya A, Fettach S, Zellou A, Cherrah Y. Ethnopharmacological study of medicinal plants used in the treatment of skin burns in occidental Morocco (area of Rabat). South African journal of botany. 2019;121:128-42.
- Benkhnigue O, Akka FB, Salhi S, Fadli M, Douira A, Zidane L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc). J Anim Plant Sci. 2014;23(1):3539-68.
- MA M. Anti-diabetic and anti-hyperlipidemic action of aqueous ethanolic extracts of mentha spicata (leaves), plumeria alba (leaves) and nymphaea alba (flowers and rhizomes). IJBPAS. 2017;6:108-24.
- Bayani M, Ahmadi-Hamedani M, Javan AJ. Study of hypoglycemic, hypocholesterolemic and antioxidant activities of Iranian Mentha spicata leaves aqueous extract in diabetic rats. Iranian Journal of Pharmaceutical Research: IJPR. 2017;16(Suppl):75.
- Al-Fartosi KG, Radi H, Al-Rekabi EA. Lipid profile of diabetic male rats treated with phenolic compounds of leaves extracts from mentha longifolia and mentha spicata. Int J Pharm Biol Med Sci. 2014;3(2):26-31.
- El-Haoud H, Boufellous M, Berrani A, Tazougart H, Bengueddour R. Screening phytochimique d’une plante medicinale: Mentha spicata L. American Journal of Innovative Research and Applied Sciences. 2018;7:226-33.
- Benedec D, Vlase L, Oniga I, Mot AC, Silaghi-Dumitrescu R, Hanganu D, et al. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Note I. Farmacia. 2013;61(2):262-7.
- Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216-26.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological research. 2001;50(6):537-46.
- Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. Journal of experimental pharmacology. 2023:51-62.
- Oloya B, Namukobe J, Ssengooba W, Afayoa M, Byamukama R. Phytochemical screening, antimycobacterial activity and acute toxicity of crude extracts of selected medicinal plant species used locally in the treatment of tuberculosis in Uganda. Tropical medicine and health. 2022;50(1):16.
- Ayoola G, Coker H, Adesegun S, Adepoju-Bello A, Obaweya K, Ezennia EC, Atangbayila T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical journal of pharmaceutical research. 2008;7(3):1019-24.
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC complementary and alternative medicine. 2012;12:1-12.
- Trivedi N, Mazumdar B, Bhatt J, Hemavathi K. Effect of shilajit on blood glucose and lipid profile in alloxan-induced diabetic rats. Indian journal of pharmacology. 2004;36(6):373-6.
- Zannah S, Islam MS, Rahman AT, Asaduzzaman M, Al Bari AA, Ali Y, et al. Antidiabetic drugs in combination with hydroxychloroquine improve glycemic control in alloxan induced diabetic rats. Pharmacology & Pharmacy. 2014;2014.
- Zehad A, Islam GJ, Rashid M, Juthy NJ, Zannah S. Antidiabetic and antihyperlipidemic activities of methanolic leaf extract of Stephania japonica in Alloxan Induced Diabetic Rats. Pharmacology & Pharmacy. 2017;8(04):109.
- Islam M, Sarwar M, Rahman A, Asaduzzaman M, Ali Y, Zannah S, Rashid M. Fenofibrate potentiates the antihyperglycemic, antidyslipidemic and hepatoprotective activity of pioglitazone in alloxan-induced diabetic rats. British Journal of Pharmaceutical Research. 2016;9(6):1-9.
- Ali H, Rahman AT, Islam S, Mamun A, Zannah S, Alam AK, et al. Combined therapy of pioglitazone and atorvastatin alleviate diabetes in rats more effectively than that of mono therapy. Pharmacology & Pharmacy. 2014;2014.
- Begum MM, Rahman AT, Islam S, Asaduzzaman M, Ali H, Zannah S, et al. Simvastatin potentiates the antihyperglycemic, antidyslipidimic and antioxidative effect of glibenclamide on alloxan-induced diabetic rats. Pharmacology & Pharmacy. 2014;5(11):1059.
- Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385-411.
- Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58(Suppl 1):31-9.
- Hussain F, Hafeez J, Khalifa AS, Naeem M, Ali T, Eed EM. In vitro and in vivo study of inhibitory potentials of α-glucosidase and acetylcholinesterase and biochemical profiling of M. charantia in alloxan-induced diabetic rat models. American journal of translational research. 2022;14(6):3824.
- Jifar WW, Debele GR, Kanfe SG, Mule CT. Evaluation of in vivo antidiabetic, antidyslipidemic and in vitro anti-oxidant activity of extract and solvent fractions of discopodium penninervum hoschst leaf in mice: normoglycemic and streptozocin-induced model. Journal of Experimental Pharmacology. 2022:317-30.
- Kumari M, Jain S, Dave R. Babul (Acacia nilotica) A potential source of tannin and its suitability in management of type II diabetes. Nutrition & Food Science. 2014;44(2):119-26.
- Kako M, Miura T, Nishiyama Y, Ichimaru M, Moriyasu M, Kato A. Hypoglycemic activity of some triterpenoid glycosides. Journal of natural products. 1997;60(6):604-5.
- Ngugi MP, Kimuni N, Ngeranwa N, Orinda O, Njagi M, Maina D, et al. Antidiabetic and safety of Lantana rhodesiensis in alloxan induced diabetic rats. 2015.
- Hamadjida A, Metechie LC, Tchiengang FDT, Otto GLN, Eteme ON, Njintang NY, Mingoas JPK. Antidiabetic potential of Hibiscus sabdariffa extract in alloxan-induced diabetic rats. GSC Biological and Pharmaceutical Sciences. 2023;23(1):193-203.
- Vergès B. Lipid disorders in type 1 diabetes. Diabetes & metabolism. 2009;35(5):353-60.
- Ohno T, Horio F, Tanaka S, Terada M, Namikawa T, Kitoh J. Fatty liver and hyperlipidemia in IDDM (insulin-dependent diabetes mellitus) of streptozotocin-treated shrews. Life sciences. 1999;66(2):125-31.
- Goldberg IJ. Diabetic dyslipidemia: causes and consequences. The Journal of Clinical Endocrinology & Metabolism. 2001;86(3):965-71.
- Rajalingam R, Srinivasan N, Govindarajulu P. Effects of alloxan induced diabetes on lipid profiles in renal cortex and medulla of mature albino rats. Indian J Exp Biol. 1993.
- Islam M, Islam MS, Zannah S, Sadik G, Rashid M. Momordica charantia (Bitter melon) in combination with metformin potentiates hypoglycemic and hypolipidemic effects in alloxan-induced diabetic rats. Bangladesh Pharm J. 2018;21(2):109-17.
- Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Current science. 2002:30-8.
- Zaman K. Medicinal plants with hypoglycemic activity. Journal of ethnopharmacology. 1989;26(1):1-55.
- Chakravarthy B, GUPTA S, Gambhir S, Gode K. PANCREATIC BETA-CELL REGENERATION-A NOVEL ANTIDIABETIC MECHANISM OF PTEROCARPUS MARSUPIUM, ROXB. Indian journal of pharmacology. 1980;12(2):123-7.
- Manickam M, Ramanathan M, Farboodniay Jahromi M, Chansouria J, Ray A. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. Journal of natural products. 1997;60(6):609-10.
- Pourmorad F, Hosseinimehr S, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. African journal of biotechnology. 2006;5(11).
- Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ, Rao CA. Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC complementary and alternative medicine. 2013;13:1-9.
- Hakkim FL, Girija S, Kumar RS, Jalaludeen M. Effect of aqueous and ethanol extracts of Cassia auriculata L. flowers on diabetes using alloxan induced diabetic rats. International Journal of Diabetes and Metabolism. 2007;15(3):100-6.
- Luc G, Fruchart J-C. Oxidation of lipoproteins and atherosclerosis. The American journal of clinical nutrition. 1991;53(1):206S-9S.
- DeFronzo RA, Goodman AM, Group MMS. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. New England Journal of Medicine. 1995;333(9):541-9.

 ETFLIN
Notification
ETFLIN
Notification






