Randomized Open Clinical Trial to Evaluate Netratarpana Efficacy in Elderly Patients with Primary Insomnia (Anidra)
by Satyajit Pandurang Kulkarni ★ , Pritam Chugule, Pallavi Satyajit Kulkarni
Academic editor: Mohd Shahezwan Abd Wahab
Sciences of Pharmacy 2(1): 13-18 (2023); https://doi.org/10.58920/sciphar02010014
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
Note: August 02, 2025: Upon post-publication review, we noted that this article reports a clinical trial but was not registered in a publicly accessible, WHO-recognized clinical trial registry as required by the journal’s Clinical Trial Registration Policy. While the study received approval from an institutional ethics committee, this does not fulfill the requirements for public trial registration as outlined by international standards (ICMJE, WHO, and COPE). The corresponding author has been informed of this issue and requested to provide retrospective registration in a recognized public registry. We are currently awaiting their response. If the issue is not resolved or no response is received within one month of the notification date (August 02, 2025), the journal will proceed with retraction of the article in accordance with our editorial and ethical policies. Update (August 04, 2025): The corresponding author has responded and acknowledged that the clinical trial was not prospectively registered. The author has formally submitted a request for retrospective registration with the Clinical Trials Registry – India (CTRI) and is currently awaiting their decision. In their explanation, the author cited a lack of awareness of mandatory registration requirements at the time the study was conducted. The trial formed part of a postgraduate academic dissertation and was classified as an academic project, not subject to regulatory registration oversight. The author further stated that neither the institutional ethics committee nor the review process highlighted the requirement for prospective registration. They emphasized that the study was ethically approved and conducted in good faith and expressed commitment to upholding ethical standards in future research. The journal will continue to monitor the outcome of the retrospective registration process. If the trial is not registered in a WHO-recognized public registry within a reasonable timeframe, the journal will proceed with appropriate editorial action in accordance with its policies and international ethical standards.
21 Dec 2022
10 Jan 2023
14 Jan 2023
16 Jan 2023
Abstract: Primary insomnia is the most prevalent health problem among the elderly in India. It is linked to musculoskeletal disorders, cardiac disorders, Chronic Obstructive Pulmonary Disease (COPD), depression, and schizophrenia. The incidence rate of primary insomnia among the elderly is 5%, and the prevalence rate is 20% or more. With an increase in the geriatric population, this prevalence rate will increase further. In Ayurveda, Netratarpana is described as a treatment modality for insomnia, but it was not studied yet. The study aimed to evaluate the efficacy of Netratarpana in Anidra (primary insomnia). In this RCT, 70 patients with Primary insomnia were randomly divided into two groups. In group A (n=35), Netratarpana was performed for 14 days, and group B (n= 35) was given Jatamansi powder 5 g at night with buffalo milk for 14 days as a comparison. The follow-up was taken on the 7th, 14th, and 21st day. The assessment was done using the Insomnia Severity Index (ISI) with the addition of Ayurvedic parameters. A total of 49 (70%) patients have an average age of 59.3 ± 3 years. There were 50 (71.4%) males and 20 (28.6 %) females. The sleep pattern was disturbed among 7 (10%), delayed among 19 (27.1%), and disturbed and delayed among 44 (62.9%). We found both Netratarpana and Jatamansi powder are effective in inducing sleep and preventing awakening during the night with a p-value of <0.05. It can be concluded that Netratarpana is effective in managing Anidra (primary insomnia), but the stability of the results needs to be confirmed by further study.
Keywords: NetratarpanaPrimary insomniaAnidraPanchakarma
Introduction
Insomnia is a common clinical condition among elderly patients. It means difficulty initiating or maintaining sleep with irritability or fatigue during wakefulness. The Diagnostic and Statistical Manual for Mental Disorders defines insomnia as difficulty initiating sleep or maintaining sleep, waking up too early or chronically nonrestorative, or having poor sleep quality at least three times per week for three months despite the opportunity for sleep. In insomnia, there is dissatisfaction with sleep quantity, quality, or both. Elevated BMR, cortisol, and adrenocorticotrophic hormone (ACTH) among insomnia patients suggest hyperarousal in insomnia (1). It causes body aches, unable to concentrate, mood disturbance, and daytime sleepiness (2). Insomnia is associated with various illnesses like arthritis, musculoskeletal pain, cardiac disorders, and chronic obstructive pulmonary disease (COPD). Insomnia is also found with various psychiatric disorders like depression and schizophrenia, which badly affect the quality of life (3). A study by A B Dahale, T S Jaisoorya, L Manoj, et al. in 2015 reported an incidence rate of 5% and a prevalence of 20% or more insomnia among the elderly. There is a large variability in the prevalence rate of Insomnia in India. It has been associated with age (older age), sex (female), marital status (widowhood), and economic status (lower financial status) (2, 3).
Ayurveda is the ancient system of medicine followed mainly in India. Ayurveda also stresses the importance of sound sleep for a healthy body and mind. There are certain Ayurvedic drugs like Jatamansi (Nardostachys Jatamansi), Tagar (Valeriana Wallichii DC), and Sarpagandha (Rauwolfia Serpentine), which are proven as efficacious in insomnia (4, 5). In Ayurveda, Panchakarma therapy is also a specialized therapy to treat various health ailments, which involves preparatory procedures like Snehana (therapeutic oleation) and Swedana (sudation therapy). It also includes main methods like Vamana (therapeutic emesis), Virechana (therapeutic purgation), Bastikarma (therapeutic enema), Nasya (medication through nasal route), Raktamokshana (blood-letting), and post-operative as dietary regimen (6, 7). VP Sivarama et al. worked on Bramhi oil Shirodhara, a Panchakarma in primary insomnia (8). In this case series, Bramhi oil Shirodhara (therapeutic oil streaming over the head) was done for 45 minutes for 5 days, and the results were assessed in Insomnia Assessment Index (ISI). The study revealed improvement in insomnia at the follow-up range of 3.85% to 69.57%. However, it was only a case series, and no comparator was included. Moreover, Shirodhara is not the treatment included in the Ayurvedic texts like Charaka Samhita, unlike the Netratarpana, which is itself mentioned in the Charaka Samhita as a treatment for insomnia.
Netratarpana (therapeutic retention of medicated liquids over the eyes) is a simple procedure carried out routinely in our hospital. This procedure holds lukewarm cow ghee over the eyeball for a certain period (9). No previous study was carried out on this topic; therefore, this study was done. In the present study, 70 patients (n=70) with primary insomnia were divided randomly into two groups. In group A, Netratarpana was performed; in group B, Jatamansi powder was administered orally. The results were assessed on Insomnia Severity Index (ISI). This study aimed to study Netratarpana and Jatamansi in treating Anidra with special reference to Primary Insomnia.
Experimental Section
Materials
Jatamansi powder was prepared in the Vasantidevi pharmacy institute, Kodoli 416114. Maharashtra, India. The drug was authenticated and standardized in this institute. The buffalo milk was taken fresh from the market.
Study Settings
This study was performed at the Panchakarma department at Yashwant charitable hospital, Kodoli – 416114, Dist. – Kolhapur, Maharashtra, India. It was done during 2019 - 2021. A flowchart of the methodology can be seen in Figure 1.
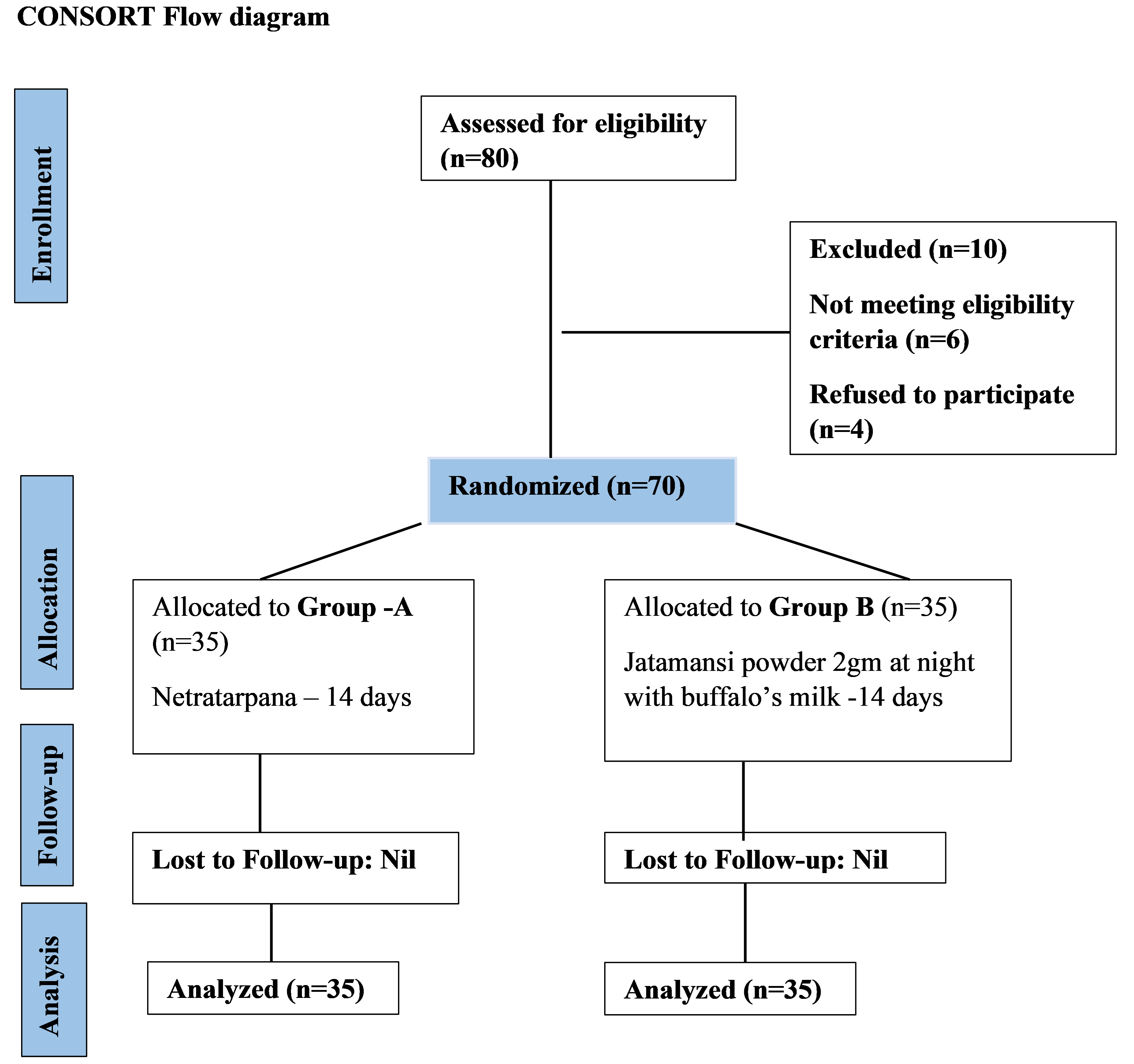
Figure 1. Flowchart of the methodology.
Ethical Considerations
The synopsis of the study was presented before the Institutional Ethics Committee (IEC) on, and the committee members' suggestions were included. An approval letter from IEC was obtained before the commencement of the trial (Ref: YAC/PG/914/2019 Dated – 26/3/2019).
Sample Size Calculation
Equation 1
where n = required sample size (n=30), z = Standard Normal Deviate (1.96), p = prevalence rate (0.019), and L = error (0.05).
Considering a dropout rate of 15 – 20%, we added 5 patients in each group, and the total sample size was adjusted to 70 (35 patients in each group). A total of 70 patients with Anidra (primary insomnia) attending the OPD of Panchakarma, fulfilling the inclusion criteria, were enrolled in this study. Before enrollment, a written consent form was filled up by the patient.
Inclusion criteria
- Age – 50 years and above
- Sex – any
- Primary insomnia for 6 months or more
Exclusion
criteria
- Patients with secondary insomnia.
- Patients taking sedatives, anti-depressants, or anti-histaminic.
- Patients with a co-existing psychiatric disorder like depression, schizophrenia, etc.
- Patients contra-indicated for Netratarpana.
Randomization and Blinding
A simple random sampling was performed using the card method for the randomization. As this study was an open randomized clinical trial, no blinding was done.
Interventions and Operational definitions
Jatamansi (Nodostrachys jatamansi) is the herb used for insomnia in Ayurveda. It is recommended in a dose of 5 g at night with milk. We used this as the previous study revealed its efficacy in insomnia (10).
Netratarpana
In this procedure, the patient was asked to remain in the relaxed supine position. An oil massage followed by hot fomentation was done over the head and neck. A dough around the eyeball was made from a paste of black gram. It was carried out during working hours, i.e., 9 AM to 2 PM, as per patients’ convenience. The melted cow ghee (approximately 34 – 35 ᵒC) was poured slowly into the dove and held for 15-20 minutes. The patient was asked to keep eyelids open so that the melted cow ghee directly interacted with the cornea. The temperature of the melted ghee was maintained by adding a small quantity of heated ghee.
After the procedure, the dough was removed, the eyes were cleaned with lukewarm water, and the patient was advised to inhale the herbal smoke. This procedure has been described in the Ayurvedic text as Ashtang Hridayam (11). The whole process was performed under aseptic precautions. The procedure is contra-indicated to an infected eye. The instruments and drugs were used separately for every patient. Biomedical waste out of the procedure was disposed of per guidelines for biomedical waste disposal.
Duration of the interventions and follow up
- Duration – 14 days
- Follow up – 7th, 14th and 21st day
Assessment criteria
The Insomnia Severity Index (ISI) scale was used to assess the effect on insomnia. To incorporate Ayurvedic parameters, we added a few questions related to the Ayurvedic parameters to Insomnia Severity Index (ISI) (12). The case records proforma (CRP) was prepared, which included demographic details of the patient, details of the intervention, and questions to assess the effect of the therapy.
The total effect of therapy was assessed considering the overall improvement in signs and symptoms. After the completion of the treatment course and follow-up period. The total effect was recorded in the following categories:
- Excellent > 75% Relief in assessment parameters.
- Moderate > 50% And < 75 % Relief in assessment parameters.
- Marginal > 25% And < 50 % Relief in assessment parameters.
- Negligible < 25% Relief in assessment parameters
Data collection and data handling
We prepared case record forms (CRFs), and the record was kept in a secure digital format. All data were entered into a computer by giving a coding system. Data entry errors were removed. Data obtained was compiled on an MS Office Excel Sheet (version 2019, Microsoft Redmond Campus, Redmond, Washington, United States). Data were subjected to statistical analysis using a Statistical package or social sciences (SPSS v 26.0, IBM). Descriptive statistics like frequencies and percentages for categorical data, Mean and SD for numerical data were depicted. The comparison of frequencies of categories of variables with groups was made using the chi-square test. The statistical significance was set to p < 0.05.
Result
We found more patients in the age group of 50-60 years (49 patients (70%)) as compared to the age group of 61-70 years (20 patients (28.5%)), and one patient (0.7%%) was above 70 years. The mean age was 59.3 years, with an SD of 3. There were 50 (71.4 %) males and 20 (28.6%) females in our study. Among the religions, Hindu 65 (92.9 %), Buddha 2 (2.9%), Jain 1 (1.4%), and Muslim 2 (2.9%) were found in this study. All the patients (n =70) were married in our study. We observed that 50 patients (71.4 %) had been involved in a physically stannous job or profession. In comparison, 7 (10 %) had a mentally stannous job or profession, and 13 (18.6 %) felt their job or career was physically and mentally stannous. In the sleep pattern, 7 (10 %) had disturbed sleep, 19 (27.1 %) had delayed sleep, and 44 (62.9 %) had experienced both disturbed and delayed sleep (see Table 1).
Table 1. General Observations.
|
Frequency (Percentage)
|
Chi-square |
p-value |
|
|
1. Age50 – 60 61 -70 > 70
|
49 (70)20 (28.5)1 (0.7)
|
---- |
------ |
|
2. Sexa) Maleb) Female
|
50 (71.4)20 (28.6)
|
0.280
|
0.597
|
|
3. Religiona) Hindub) Buddhac) Jaind) Muslim
|
65 (92.9)2 (2.9)1 (1.4)2 (2.9)
|
3.138
|
0.371
|
|
4. Marital statusa) Marriedb) Unmarried
|
70(100) 00 (0)
|
|
|
|
5. Occupationa) Physically stannousb) Mentally stannousc) Physically and mentally stannous
|
50 (71.4)7 (10)13 (18.6)
|
0.915
|
0.633
|
|
6. Sleep Patterna) Disturbedb) Delayedc) Disturbed & delayed
|
7 (10)19 (27.1)44 (62.9)
|
3.540
|
0.170
|
Among the assessment parameters, we found that sleep induction in group A (after treatment) was reduced from 3 to 1. Meanwhile, in group B, it was reduced from 3 to 0. Awakening at night was reduced from 3 to 2 in group A and from 2 to 0 in group B. The final awakening was reduced from 3 to 2 in group A and 3 to 0 in group B. The total sleep duration was also reduced score from 3 to 0. The sleep quality score was reduced from 3 to 2 in group A; in group B, the sleep quality score decreased from 3 to 0. In group A, the score was reduced from 3 to 1 in the parameter of well-being during the day. The same parameter score was reduced in group B from 3 to 0. The functioning capacity during the day was reduced from 3 to 1 in group A and from 3 to 0 in group B. The daytime sleepiness was not reduced in group A but in group B (from 2 to 0).
In the Ayurvedic parameters such as Akshi Shiro Gaurav (heaviness in the eyes and the head), Alasya and Jrumbha (laziness), Angamard (body ache), Glani (Dizziness), and Kshudhamandya (loss of appetite), the score was reduced from 3 to 1 in group A. In group B, it was reduced from 3 to 0, 2 to 0, 2 to 0, 2 to 0, and 3 to 0, respectively (see Table 2).
Table 2. Comparison of Ayurvedic parameters in groups A and B.
|
Group A
|
Z Value
|
p-Value
|
Group B
|
Z Value
|
p-Value
|
|||
|
BT
|
AT
|
BT
|
AT
|
|||||
|
1. Sleep Induction
|
3
|
2
|
-5.182
|
0.000
|
3
|
0
|
-5.176
|
0.000
|
|
2. Awakening during the night
|
3
|
2
|
-5.113
|
0.000
|
2
|
0
|
-5.160
|
0.000
|
|
3. Final awakening
|
3
|
2
|
-5.243
|
0.000
|
3
|
0
|
-5.195
|
0.000
|
|
4. Total sleep duration
|
3
|
2
|
-5.232
|
0.000
|
3
|
0
|
-5.215
|
0.000
|
|
5. Sleep Quality
|
3
|
2
|
-5.258
|
0.000
|
3
|
0
|
-5.262
|
0.000
|
|
6. Wellbeing during the day
|
3
|
1
|
-5.241
|
0.000
|
3
|
0
|
-5.262
|
0.000
|
|
7. Functioning capacity during the day
|
3
|
1
|
-5.098
|
0.000
|
3
|
0
|
-5.117
|
0.000
|
|
8. Sleepiness during the day
|
2
|
2
|
-5.069
|
0.000
|
2
|
0
|
-5.309
|
0.000
|
|
9. Akshi Shiro Gaurav
|
3
|
1
|
-5.822
|
0.000
|
3
|
0
|
-5.226
|
0.000
|
|
10. Alasya & Jrumbha
|
3
|
1
|
-5.472
|
0.000
|
2
|
0
|
-5.243
|
0.000
|
|
11. Angamarda
|
3
|
1
|
-5.215
|
0.000
|
2
|
0
|
-5.328
|
0.000
|
|
12. Glani
|
3
|
1
|
-5.445
|
0.000
|
2
|
0
|
-5.251
|
0.000
|
|
13. Kshudhamandya
|
3
|
1
|
-5.288
|
0.000
|
3
|
0
|
-5.266
|
0.000
|
Note: (AT) means after treatment and (BT) means before treatment.
Discussion
This study aimed to evaluate the efficacy of Netratarpana in primary insomnia in elderly patients. The study's secondary objectives were to evaluate and compare the efficacy of Jatamansi and Netratarpana in managing primary insomnia. We found that the Jatamansi powder and Netratarpana effectively manage primary insomnia. In previous studies, age is associated with insomnia, where elderly patients become dependent and avoid hospital visits. We found 70.4% male and 28,6% female patients in our study. Previous studies reveal that insomnia is more prevalent among females, but more males were seen in this study (p = 0.597). It is also revealed in the previous studies (3, 13) that despite a high prevalence rate of insomnia, the public often tends to ignore it. Since this study was conducted in rural areas, fewer females sought consultation. Secondly, insomnia is present only in the subclinical form in India; very few patients visit the hospital and seek treatment.
More Hindu religion (65, 92.9 %) patients were seen as compared to others (Buddha 2.9%, Jain 1.4 %, and Muslim 2.9%). The p-value is 0.371, which suggests no statistically significant association between primary insomnia and religion. The previous studies also support the same observation. In the marital status, we found all the patients were married. P Dhaval, S Joel, and P Pragnesh observed a higher prevalence of insomnia among divorced, separated, and widowed females who are more prone to suffer from insomnia (3). However, this applies only to young patients with insomnia. As we included only elderly patients in our study, this does not apply to our study.
Among the occupations, physically stannous was 71.4%, mentally stannous was 10%, and 18.6% was both. Reduced physical activity is a precipitating factor of insomnia, but in our study setting, as there were more patients from the occupations having physical work, we obtained these results (13). The p<0.05 suggests no statistically significant association between insomnia and occupation in this study.
We found patients mainly complaining of disturbed sleep were 10%, delayed sleep was 27.1%, and 62.9% for both, with p<0.05. It means there is no statistically significant association between insomnia and sleep pattern in our study settings. As insomnia is a subjective complaint, the assessment was based on the scale. There can be a variation in sleep patterns and duration. For example, one individual may feel satisfied by sleeping 4 hours, while the other may not be satisfied by even sleeping 6 hours. Insomnia has different components, like both disturbed sleep and delayed sleep.
In the parameters like sleep induction, awakening during the night, final awakening, total sleep duration, sleep quality, well-being during the day, functioning capacity during the day, and sleepiness during the day, the p-value is less than 0.05, which means that both the interventions, i.e., Jatamansi powder and Netratarpana are efficacious in the management of primary insomnia. We also found that the patients accepted both therapies. Though for Netratarpana, the patient had to visit the hospital daily, they felt safe as the procedure was applied externally only. So, we found a good acceptance for both interventions. The patients also accepted the Jatamansi powder as they thought it better than sedatives and anxiolytics of the modern system of medicine, as they have chances of dependence.
We believe various modes of action of this procedure (Netratarpana) may induce sleep. First is physical factors. The patient was asked to be in a relaxed supine position in a private therapy room. Massage and fomentation cause more relaxation and diminish stress. This may be the first step in sleep induction. The main aim of the Netratarpana is to deliver fats through the cornea. It is well-known that fat-soluble substances can be absorbed through the cornea. A study on Ayurvedic medicated ghee, Panchagavya Ghrita, showed its sedative action (14). Thus, the overall effect of Netratarpana is sleep induction and satisfaction with sleep in quantitative and qualitative terms.
Strengths & Weakness of the
study
Strength
In this study, the intervention tried was Netratarpana, an external application over the eye. Since it is an external application, less time-consuming, and effective, it was well accepted by the patients. It requires cow ghee, black gram powder, or swimming goggles, which are cheap materials available anywhere. The Netratarpana is a safe procedure and can be performed even by a trained layperson at home.
Weaknesses
The intervention applies only to primary insomnia. The interventions compared have different routes of administration. Jatamansi powder is used orally, while Netratarpana is applied over the eyeballs. Therefore, the comparison is questionable. The duration of the intervention is 14 days which appears too short to draw meaningful conclusions. The therapy period should be not less than 4 weeks, but this topic was an academic study for the completion of a post-graduate degree course; it was shortened due to technical issues. The results of this study are generalizable for those insomnia patients who are not on sedatives or anxiolytics.
Conclusion
The study concludes that the Panchakarma procedure (Netratarpana) effectively manages primary insomnia in elderly patients. However, the stability of the results needs to be confirmed by further study.
Declarations
Ethics Statement
The study and experimental design were approved by Institutional Ethical Committee (IEC) with approval letter number of YAC/PG/914/2019 Dated – 26/3/2019.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
Reference
- Dopheide JA. Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. 2020 Apr;26(4):S76–84.
- Daniel J. Buysse. Insomia. JAMA. 2013;309(7):706–16.
- Gibson-Smith D, Bot M, Milaneschi Y, Twisk JW, Visser M, Brouwer I a., et al. Insomnia Among Elderly Primary Care Patients in India. J Clin Psychiatry. 2015;77(September):22–7.
- Pundarikakshudu K, Sharma AK, Bhatt CJ, Kanaki NS. Development and validation of a high-performance thin-layer chromatographic (HPTLC) method for simultaneous quantification of reserpine, atropine, and piperine in Sarpagandha ghanvati, a classical ayurvedic preparation. J AOAC Int. 2019;102(4):1027–32.
- Toolika E, Bhat N, Shetty S. A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia). AYU (An Int Q J Res Ayurveda). 2015;36(1):46.
- Ministry of AYUSH. NAMASTE - Portal [Internet]. Goverment of India. 2019 [cited 2023 Jan 15]. Available from: http://namstp.ayush.gov.in
- Dornala SN, Ayyagari R. Guidelines for safer panchakarma practice in non-covid clinical care during corona pandemic. J Ayurveda Integr Med. 2022 Apr;13(2):100426.
- Vinjamury SP, Vinjamury M, Der Martirosian C, Miller J. Ayurvedic therapy (Shirodhara) for Insomnia: A Case Series. Glob Adv Heal Med. 2014;3(1):75–80.
- Shukla AV. Charaka Samhita. 2015th ed. Acharya Vidyadhar Shukla PR tripathi, editor. Varanasi: Krishnadas Academay; 2000 p.
- Misar Wajpeyi S, Durgaprasad Misar S, Kuchewar V. A Comparative Study of Efficacy of Jatamansi Vati and Abhyanga in Management of Anidra with Special Reference to Insomnia. 2016;(March 2016).
- Vagbhata. Ashtang Hridaya. Tripathi DB, editor. Varanasi: Chaukhambha; 2019. 1379 p.
- Smith MT, Wegener ST. Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Rheum. 2003 Oct;49(S5):S184–96.
- Gehrman P, Ancoli-Israel S. Insomnia in the elderly. Insomnia Diagnosis Treat. 2016;224–34.
- Achliya GS, Wadodkar SG, Dorle AK. Neuropharmacological actions of panchagavya formulation containing Emblica officinalis Gaerth and Glycyrrhiza glabra Linn in mice. Indian J Exp Biol. 2004 May;42(5):499–503.

 ETFLIN
Notification
ETFLIN
Notification







