An Overview of the Historical Context for Jamun's Diverse Medicinal Properties
by Tanmay Sanjay Kamble ★ , Kshitij Suhas Shirke, Kiran Babu Uppar, Sonal Balasaheb Bangar , Namrata Santosh Naware, Shreya Sakharam Ambatkar, Mukesh Patil , Ashish Jain
Academic editor: James H. Zothantluanga
Sciences of Phytochemistry 2(1): 34-45 (2023); https://doi.org/10.58920/sciphy02010042
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
29 Mar 2023
12 Apr 2023
13 Apr 2023
19 Apr 2023
Abstract: Syzygium cumini, also known as Jamun, Jambul, or Indian blackberry, is a species of tree native to the Indian subcontinent. A comprehensive literature review shows that Jamun can be considered one of the most versatile herbal medicines with antidiabetic, antioxidant, antiinflammatory, and other properties. This review aims to investigate and understand the previous research on Jamun, including its pharmacognosy and pharmacological history, to confirm its potential to treat a variety of illnesses. The study also examined the current pharmaceutical formulations available in the market to understand the potential for developing medications from the components of Jamun. To comprehend the available studies, the analytical backdrop is also reviewed. Despite being the focus of many research studies, there are still many unanswered questions regarding Jamun. Therefore, the best formulations or products may be produced in these sectors, possibly through nutraceuticals, to support improved pharmacological aspects or health promotion. This review will help identify unexplored areas where specific tasks related to Jamun can be done.
Keywords: Syzygium cuminiExtraction techniquePhytochemical screeningPharmacological activitiesFormulationAnalytical background
Introduction
Jamun is a fast-growing species that can grow to the heights of up-to 30 m (100 ft) and can live for more than 10 decades. Its lush foliage, grown solely for decorative purposes, provides shade. The bark is hard and dark grey at the tree's base and softer and lighter grey as it rises. The wood is kiln-dried, making it water-resistant and therefore useful in train sleepers and for placing motors in wells. Although it is difficult to work with in carpentry, it is occasionally used to build inexpensive furniture and homes. When young, the leaves of Jamun have a turpentine-like smell and are pinkish in colour. As they grow, they turn into a leathery, glossy dark green colour with a yellow midrib. These leaves are highly nutritious and are often fed to cattle. Between March and April, Jamun trees bloom with tiny, fragrant flowers with a diameter of 5 mm (0.2 in). The fruits of this species are referred to as "drupaceous," and they begin to form by May or June and show similarities with huge berries. The shape of fruit is ovoid and oblong. The unripe fruit is green in colour, and as it develops, it becomes pink, then bright crimson red, and finally black. There is a variety of tree that gives white fruit. Eating the fruit tends to turn the tongue purple, and it has a flavour profile that is sweet, somewhat acidic, and astringent (1).
Jamun, also known as Malabar plum, Java plum, black plum, Jaman, Jambul, or Jambolana (see Figure 1), is a poly-embryonic species belonging to the family Myrtaceae, commonly known as the Indian blackberry. The tree, Syzygium cumini, is evergreen and tropical, native to the Indian subcontinent, and naturalized in America, Africa, and Australia. The leaves, seeds, and roots of the tree can be used for numerous purposes. The fruit is violet-dark blue in colour, while the seeds are brown, and they have a sweet and bitter taste, respectively. The fruit has a slight odour and is oval in shape, measuring 1-2cm long and 0.5-1cm wide, tapering at the apex (2, 3).
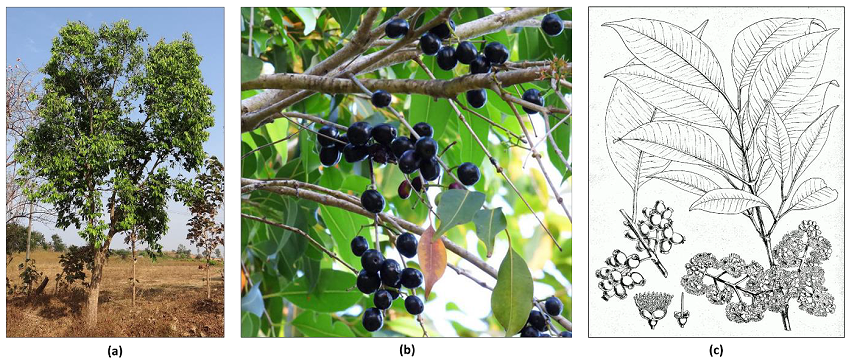
Figure 1. Images depicting an overview of S. cumini (L.) Skeels. Note: (a) Jamun trees, (b) Jamun fruits, (c) Parts of Jamun tree.
Utility of Jamun as Food
Jamun, a fruit that is native to India, can be used in various culinary ways. High-quality Jamuns can be used to make jam, tart sauces, and sauces with a sweet or acidic flavor and little to no astringency. They can become more palatable by soaking astringent fruits in salt water or poking, rubbing, and letting them stand for an hour. Jamun juice of high caliber works wonders for sherbet, syrup, and "squash," which is a bottled beverage made from crushed fruits for 5-10 minutes at 140°F, from which juice is extracted and combined with sugar and water. Preservatives like citric acid and sodium benzoate are added to the mixture. All fruits can be used to make juice, but it's advised not to press the fruit when extracting the juice from cooked Jamuns as the juice will be less stringent. The white-fleshed Jamun has sufficient pectin, and unless heating is done quickly, produces a highly stiff jelly. On the other hand, the more popular purple-fleshed Jamuns produce jelly with vibrant colors but lack pectin. Thus, they must either be combined with pectin-rich fruits like unripe or sour guavas or necessitate the addition of a commercial jellying agent. Jamun is also a significant source of port-like wine in Goa, and the fermented fruit has been used to make brandy and a distilled beverage known as "Jambava." Finally, India produces a lot of Jamun vinegar, which has a lovely clear purple color with a moderate flavor and a nice perfume (2).
Traditional Uses of Jamun
Jamun, a tree traditionally used in Ayurvedic treatment, has various parts that are utilized for medicinal purposes, including fruits, leaves, seeds, and bark. The bark has been historically used as an astringent due to its tannins and carbohydrates. The seed contains a glycoside called Jamboline, which has anti-diabetic properties, and it has also been shown to have anti-inflammatory benefits in rodents and antioxidant qualities in diabetics. The Jamun's fruit pulp and seeds have been shown to benefit diabetics by lowering blood sugar levels and avoiding problems including neuropathy and cataracts. Jamun is most commonly used as an adjuvant treatment for type 2 diabetes due to its ability to lower blood sugar levels. In the case of overproduction of glucose, the seeds contain compounds like glucosides-Jamboline and ellagic acid, which can prevent the starch to convert in sugar. The Jamun has a rich history in alternative medicine, and all of its components can be used medicinally (2).
Collection and Drying of Jamun
The study conducted by Mahalakshmi R. et al. (2022), can be referred to for carrying out the collection and drying of Jamun (4). In another experiment, Santhalakshmy S. et al. (2015) employed a spray dryer in a pilot plant with different operating conditions, drying 0.6 kilogramme of water per hour. A two-fluid nozzle, a drying chamber, two cyclone separators, a feed flow rate of 10 mL/min, a pressure range of 0.8 to 1.2 kg/cm2, and a temperature of 25 °C were all included in the spray-drying assembly. The samples of dry powder were gathered and kept in airtight containers.(5).
Extraction Techniques Used for Jamun
Extraction is the first step in isolating desired natural products from base materials. Based on the extraction principle, there are numerous extraction processes, including solvent extraction, distillation, pressing, and sublimation. Solvent extraction is the method that is most frequently used. The process of extracting natural products involves the following steps: allowing the solvent to permeate the solid matrix, allowing the solute to dissolve in the solvents, allowing the solute to diffuse out of the solid matrix, and collecting the extracted solutes (6). Any element that increases diffusivity and solubility in the above steps can make the extraction process easier. The extraction efficiency is influenced by the extraction solvent's characteristics, the material particle size, the solvent-to-solids ratio, the extraction temperature, and the extraction time (6).
The traditional extraction method has been improved over time as technology has advanced, aiming to increase yield or obtain high-quality finished goods or extract. The procedure involves separating the extract from the material, either through mechanical or chemical means, which remains the same. However, the equipment or solvent used in the extraction process may vary. For example, a microwave is utilized instead of a pressing machine in the mechanical approach, and a supercritical fluid is employed instead of the conventional hexane solvent in the chemical approach (7).
A frequently used method for extraction in the industry is solvent extraction, which has been employed in various industrial sectors such as hydrometallurgy, food engineering, pharmaceuticals, and waste treatment. Solvent extraction is a procedure that employs a chemical solvent to remove liquid from a sample of solid liquid, and both polar and nonpolar solvents can be used (7, 8). Common industrial solvents include hexane, ethanol, methanol, chloroform, diethyl ether, petroleum ether, and acetone (8).
The extraction procedure can be carried out in batch or continuous mode, and several independent parameters such as residence time, sample moisture content, extraction temperature, sample size, and choice of solvent can impact the efficiency of solvent extraction (9).
For solvent extraction, the choice of the solvent is essential. Selectivity, solubility, cost, and safety should all be taken into account when choosing a solvent. A solvent's performance is likely to be improved if its polarity values are similar to those of the solute, and vice versa, according to the similarity and inter-miscibility principle (like dissolves like). Solvent extraction studies on phytochemicals frequently use all-purpose alcohols like ethanol and methanol.
In general, the extraction process produces better results with finer particle size. This is because smaller particle size allows for improved solvent penetration and solute dispersion, resulting in increased extraction efficiency. However, particles that are too small may absorb excessive solute, leading to difficulty in subsequent filtering. High temperatures enhance both solubility and diffusion, but going too high might damage thermolabile components, remove undesired contaminants, and lose solvent. Until the solute reaches equilibrium inside and outside the solid substance, extraction efficiency increases with longer extraction times within a particular time window. The solvent-to-solid ratio boosts the extraction yield, but a ratio that is too high may produce too much extraction solvent and prolong the concentration process (10).
When discussing the extraction of Jamun seeds, it can be accomplished using the same procedure. The extraction can be carried out using water, as well as the binary solvents aqueous methanol (50% v/v) and aqueous ethanol (50% v/v). The extraction procedure can be performed using time intervals of 30, 45, and 60 minutes, with a constant temperature of 50 °C (11). Furthermore, other methods can also be tested to determine the extraction results.
As per the method performed by Arun et al. (2011), freshly collected Jamun seeds were used. The seed coat was removed by shade drying, and a coarsely ground powder was obtained. Then, 100 g of seed powder was taken. It was extracted three times with ethanol, acetone, ethyl acetate, and water using a 1:2 (w/v) material-to-solvent ratio. The extraction was carried out under constant stirring for five hours at room temperature. After each extraction, the remaining material was filtered through a muslin cloth. The filtrate was collected and then stored at 4 °C for further usage. The clear filtrate was concentrated using a rotary evaporator operating under a vacuum and low temperature (40 °C). The concentrated extracts were kept at 20 °C until further examination and dried in an oven at 60 °C (12).
According to the method performed by S. Venkateswarlu et al. (2014), S. cumini seeds were cut and dried for approximately 21 days in a dust-free environment. The dried parts were then ground into powder. Ten grams of the dry powder were combined with 100 mL of double-distilled water in a 250 mL round-bottom flask, and the mixture was refluxed for one hour at 70 °C until the solution turned a light yellowish-brown colour. The resulting extract was then cooled to room temperature and filtered using cheesecloth (13).
Furthermore, the extraction of S. cumini was also performed using a Soxhlet apparatus. The ethanolic extracts of S. cumini seeds were obtained and concentrated using a rotary vacuum evaporator to create a viscous mass. This mass was then reconstituted at a concentration of 1 mg/mL (14).
In the work conducted by Shikha Pandhi et al. (2019), the extraction of seeds of S. cumini was carried out using ultrasonication. The powdered seeds were mixed with ethanol and sonicated, followed by filtering and concentrating the extract with a vacuum rotary evaporator. Additionally, a Microwave-assisted extraction method was also performed (14).
Phytochemical Profile of Jamun
All parts of the Jamun tree, including its fruits, contain abundant amounts of different phytochemicals. Jamun fruits, for example, are rich in anthocyanin, glucosides, ellagic acid, iso-quercetin, kaempferol, myricetin, and other phytochemicals. Similarly, Jamun seeds are abundant in phytochemicals. Phytochemicals such as Jambosine, gallic acid, ellagic acid, corilagin, quercetin, and β-sitosterol. Flowers, are a good source of oleanolic acid, while tannins and gallic acid are responsible for the fruit's astringency or sourness. Additionally, the roots of the Jamun tree contain several flavonoids and glycosides (3).
Phytochemical screening was conducted for the roots, and flavonoids, glycosides, and isorhamnetin 3-O-rutinoside were reported as constituents (15). For the stem/bark phytochemical screening, the reported study showed the presence of friedelin, ellagic acid, gallic acid, gallotannin, ellagitannin, myricetin, β-sitosterol, and betulenic acid (16, 17). Additionally, bergenins (18), flavonoids, and tannins (19) were also observed. Bornyl acetate, triacontanol, n-Dotricontanol (20), quercetin, maslinic acid, betulinic acid, myricetin, n-nonacosane, and n-dotricontanol were found in the leaves, along with terpenoids observed in the screening studies reported (21). Esterase and galloyl carboxylase were also reported as present in the leaves (22). On phytochemical screening of the flowers of S. cumini, oleanolic acid, ellagic acid, iso-quercetin, kaempferol, myricetin, kaempferol, dihydro-myricetin, quercetin, and arabinoside were found (3, 23, 24).
Phytochemical screening of the fruit pulp of S. cumini showed the presence of raffinose, citric acid, fructose, gallic acid, malic acid, anthocyanin (25), delphinidin, petunidin, and malvidin (3, 26). Jamun peel powder was found to be useful as a food and drug coloring, and anthocyanin pigments from fruit peels were investigated for their antioxidant activity and stability as extracts and in formulations (26). Phytochemical screening of the seeds of S. cumini revealed the presence of fats, Jambosine, Jamboline, gallic acid, ellagic acid, corilagin, chromium, vanadium, potassium, sodium, zinc, and tannins (3, 27). Essential oils isolated from the freshly collected leaf, stem, seed, and fruits of S. cumini showed the presence of α-terpineol, myrtenol, eucarvone, muurolol, α-myrtenal, 1,8-cineole, geranyl acetone, α-cadinol, and pinocarvone (2). Trans-ocimene, cis-ocimene, β-myrcene, α-terpineol, dihydrocarvyl acetate, geranyl butyrate, and terpinyl valerate (28) were also found.
Pharmacological Activities of Jamun
S. cumini exhibits various pharmacological activities that have been proven through authenticated research. The pharmacological activities of S. cumini can be observed and are listed in Figure 2.
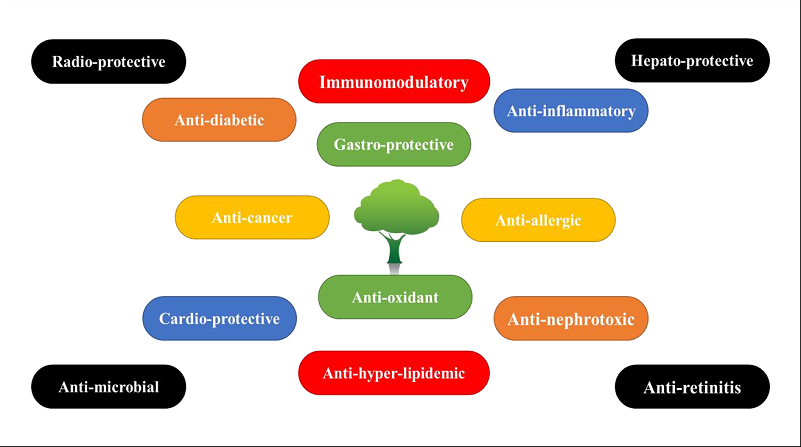
Figure 2. Overview of pharmacological actions of Jamun.
Anti-Allergic Activity
According to a study carried out by F.A. Brito et al. (2007), the antiallergic properties of aqueous leaf extracts of S. cumini (L.) Skeels (SC) were investigated. Treatments with Jamun extract at various doses were reported to reduce edema; no discernible difference was seen between the various doses utilized. Rats receiving c48/80 therapy generated histamine in their peritoneal mast cells, but pre-treatment with Jamun leaf extract (1 g/mL) stopped this allergic reaction in the mast cells. OVA administration to BALB/c mice resulted in a significant accumulation of leukocytes, mononuclear cells, and eosinophils in the pleural cavity; however, pre-treatment of these mice with Jamun leaf extract at least on before to OVA administration significantly reduced the accumulation of eosinophils in the pleural cavity, indicating the extract's anti-inflammatory action (29, 30).
The study was done by G.V. Balakrishna et al. (2016), on the antiallergic properties of aqueous, methanol, and methanol fraction of the aqueous extract of Jamun roots, these extracts prevented mast cell degranulation from causing the release of histamine, which is what led to mice experiencing clonidine-induced catalepsy. Last but not least, it was demonstrated that giving mice different root extracts of Jamun suppressed milk-induced eosinophilia (30, 31).
Anti-Cancer Activity
According to D. Barh et al. (2008), several different cell lines have been used to test various Jamun components for cytotoxic activity in vitro. By using the MTT assay, the cytotoxic effect of the crude extract from Jamun fruit skin was investigated in HeLa (HPV-18 positive) and SiHa (HPV-16 positive) cells. The crude extract was discovered to have a cytotoxic effect on both cell types. However, the HeLa cells were more significantly affected than the SiHa cells by the change. Similar to this, HeLa cells demonstrated more apoptosis when exposed to 50% methanol extract than SiHa cells (32).
In the study carried out by Li et al. (2021), nine phytochemicals overall were investigated for anticancer activities in the ovarian cancer cell line using chloroform extraction from the S. cumini fruits. Using the PA-1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium test, the 50% inhibition (IC50) concentration and cell cytotoxicity values were calculated. A cell scratch assay was used to gauge the phytochemicals' capacity to restrain growth. Cisplatin was used as a positive control. Quercetin and gallic acid were more effective at killing cells than oleanolic acid up to 5 g/mL serial doses, but only at concentrations of 2.5 g/mL and higher. Oleanolic acid, together with QC and GA, significantly yet mildly reduced cell growth (33).
With the research of Afify et al. (2011), the anticancer activity of S. cumini (L.) fruit extracts was examined utilizing leukemia cancer cell line cell viability test. Hexane, chloroform, ether, ethyl acetate, ethanol, and water extracts were used in succession and tested for anticancer efficacy. According to their findings, the ethanol extract had greater anti-leukemia activity than the others. The fruit extract of S. cumini (L.) contains phenolic components like Kaempferol 7-O-methylether and sterols like -Sitosterol, which was responsible for their anticancer potential, according to spectroscopic observations of active constituents isolated from ethanol extract (34, 35).
Mittal et al. (2014) carried out research, in vitro development, and characterization of silver nanoparticles from S. cumini (L.) fruit extract. It was discovered that the size of newly created silver nanoparticles and their size were between 10 and 15 nm. Important findings of this research included the identification of the biomolecules in charge of producing silver nanoparticles as well as the biosynthesis mechanism. The primary factor in the decrease stabilization of nanoparticles was the presence of flavonoids in S. cumini (L.). In vitro, it was seen that the nanoparticles completely destroyed Dalton lymphoma cell lines. It was discovered that silver nanoparticles (100 g/mL) could decrease the viability of Dalton lymphoma (DL) cell lines by up to 50% (36, 37).
Anti-Diabetic Activity
When the study was carried by P. Kedar et al. (1983), in New Zealand rabbits, it shown that a single intravenous injection of streptozotocin (STZ 65 mg/kg) elevated blood sugar levels to 340 mg% and was followed with weight loss, hypercholesterolemia, hypertriglyceridemia, glycolysis, and ureamia. The raised post meal (1 1/2 hours after) values of blood sugar, cholesterol, and triglyceride were considerably reduced when Jamun seed (1 g/kg) was administered orally in a casein diet, to levels equivalent to phenformin (38).
P. Stanely MainzenPrince et al. (1998), research shows the in contrast to 7.5 g/kg body weight, oral administration of 2.5 and 5.0 g/kg body weight of the Jamun seed's aqueous extract caused a considerable decrease in blood sugar and an increase in total haemoglobin. Besides, it prevents losing body weight. Moreover, the aqueous extract decreased the generation of free radicals in the tissues under study. The study comes to the conclusion that jamun seed extract has hypoglycemic qualities. The decrease in thiobarbituric acid reactive substances and increase in reduced glutathione, superoxide dismutase , and catalase demonstrate the Jamun seed extract’s antioxidant property (39).
According to the study carried by A. Bopp et al. (2009), the leaves, fruit and pods of Jamun have been used for their hypoglycaemic activity. It has been discovered that adenosine deaminase (ADA) is a crucial enzyme involved in immunological response, DNA and purine metabolism, and peptidase activity. Although it is thought that ADA is a crucial enzyme for controlling insulin bioactivity, its therapeutic relevance for diabetes mellitus is still unknown. In this study, investigation was done on the effects of leaf extract of S. cumini (L.) on the ADA activity of hyperglycaemic subjects and the activity of total ADA and its isoenzymes in serum and red blood cells (40).
Observing the study of Rekha. N et al. (2010), streptozotocin was injected intraperitoneally once to cause hyperglycaemia, which led to a considerable rise in blood sugar levels, a fall in serum insulin levels, and a reduction in body weight was seen in diabetic rats as compared with normal control rats. Blood glucose levels significantly decreased after the composite extract was administered to diabetic rats. More so than a single injection of the extract, it also dramatically increased serum insulin levels and stopped the loss of body weight. In diabetic rats not receiving treatment, hyperlipidaemia, a notable rise in lipid peroxide levels, and a concurrent decline in antioxidant enzymes were noted. Comparative to a single extract dose, composite extract therapy considerably improved these symptoms and brought them close to normal levels. This study reports that combining S. cumini pulp and Cinnamon zeylanicum bark aqueous extracts for therapeutic benefits against streptozotocin-induced diabetic condition (41).
Also, antidiabetic activity can be seen in various parts of the S. cumini. According to the study carried by Jagetia G. et al. (2017), the plant parts that showed the antidiabetic activity along with the extract type used is given in Table 1 (30).
Table 1. Antidiabetic activity reported in various parts of Jamun.
Sl. No. | Parts Used | Extract type | Species |
1 | Seed | Aqueous, Powder, Ethanol, Ethyl acetate, Methanol | Rabbits, Rat, Humans, Mice |
2 | Stem | Ethanol | Rats |
3 | Fruit pulp | Lyophilized, Aqueous, Ethanol | Rats |
4 | Leaf | Aqueous | Humans, Rats |
Anti-Microbial Activity
The antimicrobial study was done on different parts of Jamun tree. From all majorly available studies it was observed that difference part of plant shows the antimicrobial activity on different microbial species. This can be seen in following Table 2.
Table 2. Antimicrobial activity of various parts of Jamun.
Sl. No. | Part of the plant used | Species on which action is shown |
1 | Essential oils extracted from leaves | Basillus sphaericus, Basillus sphaericus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Samonella typhimurium (42). |
2 | Hydroalcoholic extract of Jamun leaves | Candida krusei, P. aeruginosa, Klebsiella pneumoniae, S. aureus, Enterococcus faecalis, E. coli, Kocuria rhizophila, Neisseria gonorrhoeae, P. aeruginosa, and Shigella flexneri (43). |
3 | The diethyl ether, methanol, and aqueous extracts of Jamun fruit | Bacillus cereus, Staphylococcus epidermidis, Micrococcus luteus and Salmonella typhi (44). |
4 | Ethanolic extract of Jamun seeds | E. coli, B. subtilis, P. aeruginosa and S. aureus (45) |
5 | Methanol extract of S. cumini seeds | Aspergillus niger, Bacillus subtilis, Proteus vulgaris, Salmonella typhii, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans and Penicillium notatum (46) |
6 | The ethanol extract of Jamun roots | S. aureus, S. epidermidis, E. coli, Streptococcus suis, Salmonella spp., and Corynebacterium diphtheriae (47) |
Anti-Oxidant Activity
In order to review the antioxidant study or work done on S. cumini, several literatures were reviewed which is shortly summarized in following Table 3.
Table 3. Antioxidant activity of various parts of Jamun.
Sl. No. | Part of the plant used | The extract used for the activity |
1 | Fruit | Anthocyanin-rich extract prepared in acidified (5% H3PO4) ethanol (48) |
2 | Leaves | 1:1 dichloromethane and methanol extract (49) |
3 | Seeds and Fruit | Acid ethanolic extracts (50) |
4 | Leaves | Aqueous extract (51) |
5 | Seed, Stem bark, and leaves | Ethanolic extract (52) |
Gastroprotective Activity
In the investigation made by Ramirez R. et al. (2003), as evidenced by lessened gastric mucosal damage, decreased free radicals, and lessened gastric mucosal ulceration, tannins isolated from the stem bark of Jamun protect against stomach ulcers in Sprague-Dawley rats caused by oral administration of HCl/ethanol (53).
Additionally, it has been shown that the ethanol extract of Jamun seeds can lessen the production of acid-pepsin and peptic ulcers in streptozotocin-induced diabetic rats as well as indomethacin- and ethanol-induced peptic ulcers (54, 55).
Formulations Developed Using Jamun
The work done on the S. cumini, in the field of pharmaceutics can be given in following review. Various pharmaceutical as well as nutraceutical formulations can be made with various parts of S. cumini. All these formulations can be formulated to show an intended therapeutic or health promoting activity and it can be seen in Table 4.
Table 4. Available formulations of Jamun.
Sl. No. | Formulation | Part of the Plant used | Activity |
1 | Polymeric Nanoparticles, Nanoparticles | Seeds | |
2 | Oral Thin Films | Seeds | Antibacterial (58) |
3 | Chewable Tablet | Seeds | Antibacterial (59) |
4 | Peel-off mask | Leaf | Antiaging, Antioxidant (60) |
5 | Gel | Leaf | Antioxidant (61) |
6 | Syrups, Paste, Sharbat, Vermicelli. | Fruit pulp & Seeds | Nutraceutical Supplement (A vitamin supplement) (62) |
7 | Maida Biscuits | Seeds | Antidiabetic (63) |
8 | Herbal Syrup | Seeds | Antidiabetic (64) |
9 | Mouth dissolving tablets | Roots | Antidiabetic (65) |
10 | Microcapsules | Seeds | Antioxidant (66) |
Pharmaceutical Analysis
According to study carried by Heba A. S. et al. (2021), by analysing the chemical make-up of the leaf essential oil using gas chromatography-mass spectrometry, 53 components, or around 91.22% of the total oil, were identified. An IC50 value of 38.15 2.09 g/mL for the tested oil against human liver cancer cells indicated a moderate cytotoxic impact. Furthermore, it showed inhibitory properties against α-amylase and α-glucosidase with IC50 values of 57.80 3.30 and 274.03 12.37 µg/mL, respectively (67).
Study done by Kaur J. et al. (2020), shows that S. cumini extract underwent accelerated and long-term stability experiments for 6 months and 30 months, respectively. To calculate the number of different markers in the extract, an HPLC-UV method was created. The technique was used to analyse all of the stability samples after being validated in accordance with ICH guideline Q2. Regarding the control, there was no discernible difference in the fingerprint of any of the stability samples. The α-glucosidase inhibitory activity of all stability samples was also found to remain significantly unchanged, with respect to control sample, which suggest that antidiabetic activity of S. cumini extract does not change with storage (68).
Branco I. et al. (2016), research examined the phenolic chemicals found in Jamun pulp to demonstrate a link between antioxidant and in vitro anti-proliferative properties, both prior to and during pasteurisation. Using UV-vis techniques, the total amounts of phenolic compounds, flavonoids, and anthocyanins were measured. By co-injecting a reference compound, the main phenolic compounds in HPLC-DAD/UV-vis were identified (69).
With the investigation of Chitnis et al. (2012), construction of a quick, simple, and effective methodology, and a thorough pharmacognostic evaluation of S. cumini seed powder was conducted for verification of the market-available Jamun formulations. The gallic acid component of tannins has also been attempted to quantify using various chromatographic and spectrophotometric methods. 10 mL of ethanol was used to extract 1gm of powder overnight; the mixture was then filtered and used for HPTLC analysis. Only 0.2 and 0.3 of the marker peaks indicated in the API (0.95 was not discovered in any of the formulations), 0.95, and 0.95 were found. After derivatization with iodine vapours for the purpose of detecting conjugated double bond chemicals, only sample A displayed significant bands. Toluene: Ethyl was discovered to be the suitable mobile phase. Acetate: Formic Acid among the substances tested. HPTLC results showed similar Rf values in market formulations and standard gallic acid (70).
Gajera H.P. et al. (2017), conducted the research were seven different phenolics were measured using an HPLC-PDA method. Gallic, catechin, ellagic, ferulic, and quercetin were found in higher concentrations in the seed and BJLR-6, but gallic acid and catechin were found in larger concentrations in the pulp as α-amylase inhibitors. The fruit extracts concentration that display a 50% inhibition of porcine pancreatic α-amylase (PPA) activity is indicated by the IC50 value. When compared to normal acarbose (24.7 lg mL-1), the seed and kernel of BJLR-6 suppressed PPA at substantially lower concentrations, making them promising candidates for antidiabetic herbal formulations (71).
By method opted by F. Aqil et al. (2012), extracts from the pulp and seeds of the Jamun were examined on a ShimPack reverse phase column. Different gradients of 3.5% v/v aqueous phosphoric acid and acetonitrile were used in two different tests. Solvent was present in the initial linear gradient. A 95% initial for 40 minutes, 40% for 41 to 61 minutes, and then 95% in acetonitrile with a 0.75 mL/min flow rate. With breaks in between the injections, the overall runtime was 61 minutes. At 520 nm, anthocyanidins were observed. In the second gradient, solvent A was originally present at 90% for 0–5 minutes, 85% at 10 minutes, 80% at 15 minutes, 70% at 24 minutes, 62% at 35 minutes, 94% at 40–43 minutes, and ultimately 90% at 45 minutes. Other polyphenolics and ellagitannins were observed at 366 and 280 nm, respectively (50).
Conclusion
In accordance to whole review of the literature, S. cumini (Jamun) is said to be one of the most versatile herbal medicines that can be utilised as a whole. Almost every portion of the plant can be used to demonstrate potential therapeutic effects on a range of illnesses. The historical background of Jamun is being discussed, and we can draw the conclusion that it is an ancient medicinal plant that has been used for centuries in India and other regions. This conclusion is supported by traditional and scientific research, which has led to a growing understanding of Jamun's therapeutic effects. It is also being debated how to extract different active components from Jamun, and this information can be used for future studies to explore the potential of this adaptable herbal medication and look for a more practical way to extract and use its active components. In order to determine the effectiveness of Jamun and its ingredients in treating different medical diseases, investigations of phytochemical screening are also being done. This was investigated in order to understand the previous work. Additionally, the Jamun's pharmacological history was investigated in order to confirm the research on its ability to treat a variety of illnesses. To comprehend the potential for creating medications from the components of Jamun, pharmaceutical formulations that are currently on the market were examined. To comprehend the available study, the analytical backdrop is also reviewed. Despite the fact that Jamun has been the subject of substantial research, many questions remain unanswered. The best formulations or products that would support improved pharmacological aspects or health promotion might thus be developed in these fields, perhaps through nutraceuticals.
Declarations
Acknowledgment
I want to express my gratitude to all of the co-authors for their assistance with the article's preparation. I would especially like to thank Prof. Mukesh S. Patil for all of his assistance and direction during the review process. Also, I would like to show my gratitude to Principal Dr. Ashish Jain and Management of D.D. Vispute College of Pharmacy and Research Centre.
Ethics Statement
Not applicable.
Data Availability
Not applicable.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Jambolan. Available from: https://hort.purdue.edu/newcrop/morton/jambolan.html. Accessed: 9 January 2023.
- Ramteke V, Kurrey V, Kar S. Jamun A traditional fruit and medicine. Pop Kheti. (2015) 3:188–90.
- Sahu PP, Behera L, Nayak S, Samal KC. Health benefits of Jamun (Syzygium cumini) an Underutilised fruit: A ray in nanotechnology field. Journal of Pharmacognosy and Phytochemistry 2020; Sp 9(5): 74-80.
- Deore SL, Khadabadi SS, Baviskar BA. Pharmacognosy and phytochemistry: a comprehensive approach. 2nd ed. Hyderabad: PharmaMed Press; 2019.
- Shah BN, Seth AK. Textbook of pharmacognosy and phytochemistry. Second edition. CBS edition. New Delhi: CBS Publishers & Distributors Pvt Ltd; 2017.
- Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. (2018) 13(1):20.
- Geow CH, Tan MC, Yeap SP, Chin NL. A Review on Extraction Techniques and Its Future Applications in Industry. Eur J Lipid Sci Technol. (2021) 123(4):2000302.
- Sudhagar Mani, Jaya Sundaram, L. Narayanan. Solvent Extraction of Oil from Moringa (Moringa oleifera). In: 2004, Ottawa, Canada August 1 - 4, 2004. American Society of Agricultural and Biological Engineers; 2004. Available from: http://elibrary.asabe.org/abstract.asp?JID=5&AID=16940&CID=can2004&T=1. Accessed: 19 January 2023.
- Avram M, Stoica A, Dobre T, Stroescu M. Extraction of vegetable oils from ground seeds by percolation techniques. UPB Sci Bull Ser B. (2014) 76(2):13–22.
- Ćujić N, Šavikin K, Janković T, Pljevljakušić D, Zdunić G, Ibrić S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. (2016) 194:135–42.
- Raza A, Shahzad N, Qasrani SA, Sharif MN, Akram MN, Ali MU. Extraction of Bioactive Components from the Fruit and Seed of Jamun (Syzygium cumini) Through Conventional Solvent Extraction Method. Env Sci. (2015) 15(6): 991-996.
- Arun R, Prakash MVD, Abraham SK, Premkumar K. Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J Ethnopharmacol. (2011) 134(2):329–33.
- Venkateswarlu S, Natesh Kumar B, Prasad CH, Venkateswarlu P, Jyothi NVV. Bio-inspired green synthesis of Fe3O4 spherical magnetic nanoparticles using Syzygium cumini seed extract. Phys B Condens Matter. (2014) 449:67–71.
- Pandhi S, Poonia A. Phytochemical screening of Jamun seeds using different extraction methods. The Pharma Innovation Journal (2019) 226–31.
- Vaishnava MM, Tripathi AK, Gupta KR. Constituents of Cassia fistula roots. Fitoterapia. (1993) 64(1):93.
- Bhargava KK, Dayal R, Seshadri TR. Chemical components of Eugenia jambolana stem bark. Curr Sci. (1974) 645-646.
- Chaturvedi A, Kumar MM, Bhawani G, Chaturvedi H, Kumar M, Goel RK. Effect of ethanolic extract of Eugenia jambolana seeds on gastric ulceration and secretion in rats. Indian J Physiol Pharmacol. (2007) 51(2):131–40.
- Kopanski L, Schnelle G. Isolation of Bergenin from Barks of Syzygium cumini. Planta Med. (1988) 54(6):572.
- Bhatia IS, Bajaj KL. Chemical constituents of the seeds and bark of Syzygium cumini. Planta Med. (1975) 28(4):346–52.
- Craveiro A.A., Andrade C.H.S., Matos F.J.A., Alencar J.W., Machado M.I.L. Essential oil of Eugenia jambolana [Brazilian plant]. J Nat Prod. (1983) 46(4):591–592.
- G.s G, D.p S. Triterpenoid and other constituents of Eugenia jambolana leaves. Phytochemistry. (1974) 13(9): 2013-2014.
- Bhatia IS, Sharma SK, Bajaj KL. Esterase & galloyl carboxylase from Eugenia jambolana (Lam.) leaves. Indian J Exp Biol. (1974) 550-552.
- Ayyanar M, Subash-Babu P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. (2012) 2(3):240–6.
- de Carvalho Bernardo WL, Boriollo MFG, Tonon CC, da Silva JJ, Cruz FM, Martins AL, et. al. Antimicrobial effects of silver nanoparticles and extracts of Syzygium cumini flowers and seeds: Periodontal, cariogenic and opportunistic pathogens. Arch Oral Biol. (2021) 125:105101.
- Jain M, TR S. Anthocyanins of Eugenia jambolana fruits. Indian J. Chem. (1975) 13(1): 20-23.
- Veigas JM, Narayan MS, Laxman PM, Neelwarne B. Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of Syzygium cumini Skeels. Food Chem. (2007) 105(2):619–27.
- Chopra RN, Nayar SL, Chopra IC, Asolkar LV, Kakkar KK, Chakre OJ, et. al.. Glossary of Indian medicinal plants; Supplement. New Delhi: Council of Scientific & Industrial Research; 1956. 3 p.
- Vijayanand P, Jagan Mohan Rao L, Narasimham P. Volatile flavour components of Jamun fruit (Syzygium cumini L). Flavour Fragr J. (2001) 16(1):47–9.
- Brito FA, Lima LA, Ramos MFS, Nakamura MJ, Cavalher-Machado SC, Siani AC, et. al.. Pharmacological study of anti-allergic activity of Syzygium cumini (L.) Skeels. Braz J Med Biol Res. (2007) 40:105–15.
- Jagetia GC. Phytochemical Composition and Pleotropic Pharmacological Properties of Jamun, Syzygium Cumini Skeels. J Explor Res Pharmacol. (2017) 2(2):54–66.
- Balakrishna G, Sowmya K, Bollapalli VR, Mv R, Morusupalli R. Anti-Allergic Studies of Albizzia Lebbeck and Syzygium Cumini (L-Syzygium Gambolana). Open Access Journal of Microbiology & Biotechnology (2016) 1(1): 000103.
- Barh D, Viswanathan G. Syzygium cumini inhibits growth and induces apoptosis in cervical cancer cell lines: a primary study. Ecancermedicalscience. (2008) 83(2):1-10.
- Li L, Mangali S, Kour N, Dasari D, Ghatage T, Sharma V, et. al. Syzygium cumini (Jamun) fruit-extracted phytochemicals exert anti-proliferative effect on ovarian cancer cells. J Cancer Res Ther. (2021) 17(6):1547.
- Pradhan M. Phytochemistry, Pharmacology and Novel Delivery Applications of Syzygium cumini (L.). Ijppr.Human. (2016) 7(1): 659-675.
- Afify AEMMR, Fayed SA, Shalaby EA, El-Shemy HA. Syzygium cumini (pomposia) active principles exhibit potent anticancer and antioxidant activities. Afr J Pharm Pharmacol. (2011) 5(7):948–56.
- Mittal AK, Bhaumik J, Kumar S, Banerjee UC. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J Colloid Interface Sci. (2014) 415:39–47.
- Ahmad N, Nawab M, Kazmi MH. Medicinal Potential of Jamun (Syzygium cumini Linn): A Review. J Drug Deliv Ther. (2019) 9(5):175–80.
- Kedar P, Chakrabarti CH. Effects of jambolan seed treatment on blood sugar, lipids and urea in streptozotocin induced diabetes in rabbits. Indian J Physiol Pharmacol. (1983) 27(2):135–40.
- Prince PSM, Menon VP, Pari L. Hypoglycaemic activity of Syzygium cumini seeds: effect on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol. (1998) 61(1):1–7.
- Bopp A, De Bona K s., Bellé L p., Moresco R n., Moretto M b. Syzygium cumini inhibits adenosine deaminase activity and reduces glucose levels in hyperglycemic patients. Fundam Clin Pharmacol. (2009) 23(4):501–7.
- Rekha N, Balaji R, Deecaraman M. Antihyperglycemic and antihyperlipidemic effects of extracts of the pulp of Syzygium cumini and bark of Cinnamon zeylanicum in streptozotocin-induced diabetic rats. J Appl Biosci. (2010) 28:1718–30.
- Shafi PM, Rosamma MK, Jamil K, Reddy PS. Antibacterial activity of Syzygium cumini and Syzygium travancoricum leaf essential oils. Fitoterapia. (2002) 73(5):414–6.
- Oliveira GF de, Furtado NAJC, Silva Filho AA da, Martins CHG, Bastos JK, Cunha WR, et. al. Antimicrobial activity of Syzygium cumini (Myrtaceae) leaves extract. Braz J Microbiol. (2007) 38:381–4.
- Patel PR, Rao TVR. Antibacterial activity of underutilized fruits of jamun (Syzygium cumini L. Skeels). International Journal of Current Pharmaceutical Research. (2012) 4(1): 36-39.
- Meshram GA, Yadav SS, Shinde D, Singh D, Patil B. Antibacterial Study and Effect on Glucoamylase in Vitro of Aqueous and Methanolic Extracts of Syzygium cumini Seeds. Biosci Biotechnol Res Asia. (2016) 7(1):297–300.
- Mathur A, Purohit R, Mathur D, Prasad G, Dua VK. Pharmacological investigation of methanol extract of Syzigum cuminii seeds and Crateva nurvula bark on the basis of antimicrobial, antioxidant and anti-inflammatory properties. Der Chemica Sinica. (2011) 2(1): 174-181.
- Mueller M, Kheawfu K, Puttipan R, Unger frank michael, Viernstein H, Okonogi S. Anti-inflammatory, antibacterial, and antioxidant activities of Thai medicinal plants. International Journal of Pharmacy and Pharmaceutical Sciences. (2015) 7:123–8.
- Faria AF, Marques MC, Mercadante AZ. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chem. (2011) 126(4):1571–8.
- Jagetia GC, Shetty PC, Vidyasagar MS. Inhibition of Radiation-Induced DNA Damage by Jamun, Syzygium cumini, in the Cultured Splenocytes of Mice Exposed to Different Doses of γ-Radiation. Integr Cancer Ther. (2012) 11(2):141–53.
- Aqil F, Gupta A, Munagala R, Jeyabalan J, Kausar H, Sharma RJ, et. al.. Antioxidant and Antiproliferative Activities of Anthocyanin/Ellagitannin-Enriched Extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr Cancer. (2012) 64(3):428–38.
- Chanudom L, Tangpong J. Anti-Inflammation Property of Syzygium cumini (L.) Skeels on Indomethacin-Induced Acute Gastric Ulceration. Gastroenterol Res Pract. (2015) 2015: e343642.
- Mubassara S, Biswas KK, Hasan M, Hossain I, Paul S. In vitro Phytochemical, Antibacterial and Antioxidant Analyses in Different Plant Parts of Syzium cumini. International Journal of Pharmacognosy and Phytochemical Research. (2015) 7(1): 150-155.
- Ramirez RO, Roa CC. The gastroprotective effect of tannins extracted from duhat (Syzygium cumini Skeels) bark on HCl/ethanol induced gastric mucosal injury in Sprague-Dawley rats. Clin Hemorheol Microcirc. (2003) 29(3–4):253–61.
- Chaturvedi A, Bhawani G, Agarwal PK, Goel S, Singh A, Goel RK. Antidiabetic and antiulcer effects of extract of Eugenia jambolana seed in mild diabetic rats: study on gastric mucosal offensive acid-pepsin secretion. Indian J Physiol Pharmacol. (2009) 53(2):137–46.
- Jonnalagadda A. Combined Effect of Syzygium cumini Seed Kernel Extract with Oral Hypoglycemics in Diabetes Induced Increase in Susceptability to Ulcerogenic Stimuli. J Diabetes Metab. (2012) 4(1): 1000236.
- Bitencourt PER, Ferreira LM, Cargnelutti LO, Denardi L, Boligon A, Fleck M, et. al.. A new biodegradable polymeric nanoparticle formulation containing Syzygium cumini: Phytochemical profile, antioxidant and antifungal activity and in vivo toxicity. Ind Crops Prod. (2016) 83:400–7.
- Bitencourt PER, Cargnelutti LO, Stein CS, Lautenchleger R, Ferreira LM, Sangoi M, et. al.. Nanoparticle formulation increases Syzygium cumini antioxidant activity in Candida albicans-infected diabetic rats. Pharm Biol. (2017) 55(1):1082–8.
- Palakurthi SS, Jakka D, Pinnamraju DN. Preparation and Evaluation of Oral Thin Films of a Natural Product: Syzygium cumini seed powder. J Drug Deliv Ther. (2022) 12(1-S):64–70.
- Palakurthi SS, Jakka D, Singh H, Bollavaram S, B S, Pinnamraju DN, et. al.. Preparation and Evaluation of Chewable Tablets of Syzygium cumini Seed Powder. J Drug Deliv Ther. (2020) 10(3):58–64.
- Asmiati E, Rahmasari D, Winata DA, Prinastiti IN, Sari MK, Indah RA, et. al.. Formulation of Peel-Off Masks Containing Duwet Leaf Extract (Syzygium Cumini). KnE Med. (2022) 350–71.
- Gel Formulation of Jamblang Leaf Extract (Syzygium cumini L) Skeel and Antioxidant Activity. Orient J Chem. (2020) 36(5):946–53.
- Mahalakshmi R, Devisri S, Wins J, Ganguly P, Chawla N, Ahamed MAI. Formulation of value-added products from jamun seed without loss in the physicochemical and medical properties. Turkish Journal of Physiotherapy and Rehabilitation (2022) 32:3143–9.
- Kalse S, Swami S, Sawant A, Thakor N. Development and Quality Evaluation of Jamun Seed Powder Fortified Biscuit Using Finger Millet. J Food Process Technol. (2016) 7(11): 1000633.
- Kumar DMS, Valarmathi DS, Sathish DR, Naveena E, Pavithra D, Nivash P, et. al. Formulation and Evaluation of Polyherbal Syrup with Anti- diabetic activity. International Journal of Pharmaceutical Research and Applications. (2022) 7(1): 867-872.
- Farhana N. Formulation and development of mouth dissolving tablets of isolated molecules and evaluation for anti-hyperglycemic activity. Research J. Pharm. and Tech. (2014) 7(3): 275-283.
- Abdin M, Salama MA, Riaz A, Akhtar HMS, Elsanat SY. Enhanced the entrapment and controlled release of Syzygium cumini seeds polyphenols by modifying the surface and internal organization of Alginate‐based microcapsules. J Food Process Preserv. (2021) 45(1): e15100.
- El-Nashar HAS, Eldehna WM, Al-Rashood ST, Alharbi A, Eskandrani RO, Aly SH. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium cumini (Pamposia) Grown in Egypt: Chemical Characterization and Molecular Docking Studies. Molecules. (2021) 26(22):6984.
- Kaur J, Bansal G. WHO prescribed shelf-life assessment of Syzygium cumini extract through chromatographic and biological activity analyses. J Ayurveda Integr Med. (2020) 11(3):294–300.
- Branco IG, Moraes ICF, Argandoña EJS, Madrona GS, dos Santos C, Ruiz ALTG, et. al. Influence of pasteurization on antioxidant and in vitro anti-proliferative effects of jambolan (Syzygium cumini (L.) Skeels) fruit pulp. Ind Crops Prod. (2016) 89:225–30.
- Chitnis KS, Palekar SB, Koppar DR, Mestry DY. Evaluation of Syzygium cumini linn. Seed formulations available in the market using spectrophotometric and chromatographic techniques. International Journal of Pharmaceutical Sciences and Research. (2011) 3(2): 556-560.
- Gajera HP, Gevariya SN, Hirpara DG, Patel SV, Golakiya BA. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black Jamun (Syzygium cumini L.) landraces. J Food Sci Technol. (2017) 54(10):3180–91.

 ETFLIN
Notification
ETFLIN
Notification







