Antibacterial and Antifungal Activities of Pandanus amaryllifolius Leaf Extract, Fractions, and Isolate and Their Role in Anti-Dandruff Shampoo Optimization
by Rina Wijayanti ★ , Windi Susmayanti, Dias Feni Meliana, Afifah Husnun Fauziyah, Aprilia Mega Anjeline, Devyra Yunika Mutiara Sari Suwarto Putri, Fadhia Tafrichatul Ulya
Academic editor: Adeleye Ademola Olutayo
Sciences of Pharmacy 4(2): 51-65 (2025); https://doi.org/10.58920/sciphar0402299
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
16 Dec 2024
01 Jan 2025
06 Jan 2025
08 Apr 2025
Abstract: Pandan leaves (Pandanus amaryllifolius Roxb.) possess various biological activities due to their secondary metabolites, including flavonoids, alkaloids, phenolics, saponins, and triterpenoids/steroids. This study aimed to evaluate the antibacterial and antifungal activities of pandan leaf extracts, fractions, and isolates against Staphylococcus aureus, Escherichia coli, and Pityrosporum ovale, as well as to optimize surfactants in shampoo formulations. The experimental design used a post-test-only control group. Samples were processed through maceration, followed by fractionation and isolation using the KLT-P method. Antibacterial and antifungal activities were assessed using the agar well diffusion method. The most effective antibacterial activity against E. coli was observed in the n-hexane isolate at 700 ppm, with an inhibition zone of 19.6 mm, classified as strong. For S. aureus, the 100% water fraction produced an inhibition zone of 9.96 mm, classified as weak. The ethanol extract showed antifungal activity against P. ovale with an inhibition zone of 6.29 mm, categorized as moderate. Shampoo formulation optimization using sodium lauryl sulfate and cocamide DEA resulted in an ideal ratio of 0.235 to 0.764. This combination produced a shampoo with acceptable physical characteristics, including a pH of 5.71, foam height of 2.56 cm, and viscosity of 899.9 cps, all of which met the standard requirements.
Keywords: Pandan isolatesAntibacterialAntifungal activity
Introduction
The common main health problem is infectious diseases caused by Staphylococcus aureus,Escherichia coli, and Pityrosporum ovale. The most common infectious diseases are gastrointestinal tract infections such as diarrhea, skin infections such as furuncles, and scalp infections such as dandruff. Diarrhea causes 9% of deaths in children under the age of 5 years and is the second leading cause of death globally. Diarrhea is caused by Escherichia coli and Staphylococcus aureus bacteria (1). Staphylococcus aureus is a major human pathogen that can adapt to various hosts and environmental conditions and can cause many infections(2). The prevalence of infection due to Staphylococcus aureus bacteria in Indonesia is estimated to reach 28%, and around 20% of these bacteria are found on the surface of human skin. The presence of abnormal activity and the number of Staphylococcus aureus bacteria on the skin can cause several skin diseases, namely folliculitis, furuncles, impetigo, and wound infections (3) besides bacteria, fungi such as Pityrosporum ovale on the scalp which causes dandruff. Dandruff is an anomalous condition of the scalp due to an excessive stratum corneum layer, causing the scalp to become dirty and smelly (4). The prevalence of dandruff in Indonesia is ranked fourth after China, India, and the USA. Dandruff becomes a problem for up to 50% of the global population, especially during puberty and adolescence, but it is undeniable that it often occurs in children (5).
Dandruff is primarily caused by excessive activity of the scalp’s sweat glands, which produce metabolites that contribute to flaking and irritation. This condition is further aggravated by the presence of the yeast Pityrosporum ovale, a microorganism commonly associated with scalp infections(6).Although antibiotics are often prescribed to manage such infections, their prolonged use has led to serious concerns, particularly antibiotic resistance. This resistance can arise through several mechanisms, including enzymatic degradation of antibiotics, altered membrane permeability, modification of drug-binding sites, or reduction in drug receptor availability on bacterial cells. In response to this growing challenge, researchers have turned to medicinal plants as alternative antimicrobial agents. Among them, pandan leaves (Pandanus amaryllifolius Roxb.) have shown promising pharmacological potential. Traditionally used in Southeast Asia, pandan leaves are known for their antibacterial, antifungal, antioxidant, antidiabetic, and anticancer properties. These activities are attributed to their rich content of bioactive compounds such as alkaloids, flavonoids, saponins, tannins, polyphenols, dyes, and essential oils (7, 8). Previous studies have explored pandan leaf extracts, fractions, and oils for their antimicrobial effects (9-12). However, comprehensive evidence identifying which specific compounds contribute to these activities remains limited.
Based on these concerns, this study was conducted to explore pandan leaves (Pandanus amaryllifolius Roxb.) as a potential source of antibacterial and antifungal agents. In this study, we evaluated the antimicrobial activity of pandan leaf extracts, fractions, and isolates against Staphylococcus aureus, Escherichia coli, and Pityrosporum ovale. We also formulated an anti-dandruff shampoo using the active extract and optimized its surfactant composition to ensure effective cleansing, stability, and safety (13). This research aims to support the development of herbal-based alternatives to conventional antimicrobials, particularly for managing skin, scalp, and gastrointestinal infections.
Methodology
Materials
Plant Identification
Determination Letter issued by the Pharmacy Laboratory of Sultan Agung Islamic University Semarang No: 007/B.1./SA-F/II/2024. Identification was conducted by looking at the taxonomic key containing the characteristics. These characteristics were then arranged sequentially and appropriately using the taxonomic key by adjusting the characteristics of the observed plant until the identity of the plant was obtained.
Extraction
Pandan leaves were dried in a cabinet, cut into small pieces, and blended until powdered. Then, Pandan powder was weighed and put into a glass jar. Furthermore, Pandan powder was soaked in 96% ethanol with a ratio of 1:10 for three days, then filtered and obtained the filtrate. The filtrate was then evaporated in a rotary evaporator until thick and placed in a sterile glass container.
Phytochemical Screening
Flavonoid Test
The extract of 2 mL of Pandan leaves (Pandanus amaryllifolius Roxb.) was dissolved in 2 mL of HCl 2 N added with Zn powder. If an orange precipitate is formed, it contains flavonoid compounds (14).
Saponin Test
200 mg of Pandan leaves (Pandanus amaryllifolius Roxb.) thick extract was dissolved in 5 mL distilled water, which was then heated in a 60 °C oven for 10 min and shaken vigorously. If foam forms for at least 30 s (does not disappear), it contains saponin compounds (15).
Alkaloid Test
100 mg of Pandan leaves (Pandanus amaryllifolius Roxb.) extract was evaporated on a porcelain cup inside a water bath to obtain a residue. The residue was then dissolved with 5 mL of 2N HCl. The solution was then cooled, and 1 mL of the solution was put into a test tube with the addition of Dragendorff's reagent. If an orange or red precipitate is formed, it contains alkaloid compounds (16).
Phenolic Test
A thick extract of 2 mL of Pandan leaves (Pandanus amaryllifolius Roxb.) was dissolved in 2 mL of FeCl3 1%. If a green, red purple, blue or dark black precipitate is formed, it contains phenol compounds (14).
Triterpenoid or Steroid Test
A thick extract of pandan leaves (Pandanus amaryllifolius Roxb.) was dissolved in 0.5 mL of chloroform and heated. Then, 1 mL of the solution was transferred into a test tube and treated with Liebermann-Burchard reagent. The formation of a purple precipitate indicates the presence of triterpenoid compounds, while a green precipitate indicates the presence of steroid compounds (15).
Fractionation
Fractionation was carried out using the liquid-liquid extraction method. 5 g of Pandan leaves (Pandanus amaryllifolius Roxb.) extract was dissolved in 25 mL of 96% ethanol, then added with 25 mL of distilled water. Then, the solution was put into a separating funnel, and 75 mL of n-hexane was added. The solution was shaken, and the tap cover was opened occasionally to release air into the separating funnel. The separating funnel was left until the two phases separated. The n-hexane phase was on top, and the ethanol-water phase was on the bottom. Then, the two phases were separated, and the ethanol-water phase was re-fractionated using n-hexane until a clear n-hexane phase was obtained. The ethanol-water fraction was put into a separating funnel and re-fractionated using 75 mL of ethyl acetate. The solution was shaken and left until the two phases separated. The ethyl acetate phase will be on top, and the ethanol-water phase will be on the bottom (17).
Isolation
Isolation was carried out using the TLC method with preparative plates. The TLC plate was prepared by weighing 50 g of silica gel GF 254 to be dissolved with distilled water and shaken until homogeneous. The solution was then put into a 20 x 20 cm glass plate mold and left to dry in the open air. The plate was activated by heating in an oven at 120 °C for 1-2 h. The n-hexane fraction was dropped using a capillary tube at a distance of 1 cm from the end of the preparative TLC plate and saturated with ethyl acetate and n-hexane eluent (3:7). After the eluent had spread to the upper limit of the plate, the TLC plate was lifted and dried by leaving it in the open air. The stain was observed under 254 and 366 nm UV light. The stain was then scraped and dissolved with a mixture of ethyl acetate and n-hexane solvents until dry
Isolate Purity Test
By trial and error, the isolates obtained were eluted with 3 different eluents from polar to nonpolar. Furthermore, under UV light, the number of stains was counted. If the number of stains is 1, it can be said that the isolate is pure.
Antibacterial Activity of Ethanolic Extract, Ethyl Acetate Fraction, and Ethyl Acetate Isolate on Escherichia coli
Preparation of NA Media
2.3 g of Nutrient Agar (NA) was dissolved into 100 mL of distilled water in an Erlenmeyer flask. Furthermore, a magnetic stirrer heated NA on a hot plate until boiling and homogenized. NA was then sterilized by autoclaving at a temperature of 121 °C and a pressure of 2 atm for 15 min (18).
Bacterial Cultivation
The test bacteria were cultured by scratching the loop needle containing Escherichia coli bacteria aseptically, then planted on nutrient agar (NA) and incubated for 24 hours at 37 °C in an incubator (19).
Preparation of MHA Media
3.8 g of Mueller Hinton agar (MHA) was dissolved into 100 mL of distilled water. Furthermore, MHA was heated on a hot plate until boiling. MHA was then sterilized by autoclaving at a temperature of 121 °C for 25 min. The sterile MHA solution was left until the temperature dropped to 40 °C, then placed in a Petri dish that had been previously sterilized (19).
Preparation of Bacterial Suspension
Escherichia coli bacteria were taken with a loop needle and suspended in a sterile test tube containing 0.9% NaCl solution. The bacterial suspension was then vortexed until homogeneous, and the McFarland standard was used to compare its turbidity or equivalent to 1 x 108 CFU/mL (19).
Preparation of Test Solution
Test solutions of the ethanolic extract, ethyl acetate fraction, and pandan leaf isolate were prepared at a concentration of 1000 ppm as stock solutions. Subsequently, 600 ppm and 800 ppm solutions were prepared by diluting the stock solution—specifically, by taking 1.2 mL and 1.6 mL of the stock solution and diluting each with 2 mL of 10% DMSO. As a positive control, 3 mg of chloramphenicol antibiotic was dissolved in 1 mL of 10% DMSO, while the negative control consisted of 1 mL of 10% DMSO (20).
Antibacterial Activity Test
The antibacterial activity test was conducted using the agar well diffusion method against Escherichia coli. Sterile media inoculated with E. coli were punctured aseptically using a sterile cork borer to create wells with a depth of 6 mm. Test solutions at 600 ppm and 800 ppm, along with positive and negative controls, were added to the wells. The plates were then incubated at 37 °C for 24 h. The formation of a clear inhibition zone around the wells indicated antibacterial activity.
Antibacterial Activity of Water Fraction on Escherichia coli and Staphylococcus aureus
Preparation of Concentrate
Ethanol extract and water fraction were made into test solutions in concentrations of 70%, 80%, 90% and 100% by weighing 3.5 g, 4 g, 4.5 g and 5 g of sample and then diluted with 10% DMSO solvent until the volume was 5 mL (18).
Preparation of Positive Control
The solution was made by dissolving 0.01 g of chloramphenicol in 1 mL of 10% DMSO (21).
Preparation of 1% BaCl2
1 g of BaCl2 was dissolved into a 100 mL measuring flask with distilled water to the mark and then homogenized. The mixture was transferred into a tightly closed and dark reagent bottle and then stored in the refrigerator (22).
Preparation of 1% H2SO4
50 mL of distilled water was put into a 100 mL measuring flask. A 1.02 mL solution of concentrated H2SO4 was put into the measuring flask, and distilled water was added again. The solution was transferred into a closed reagent bottle and then stored at room temperature (22).
Preparation of McFarland 0.5 Solution
0.05 mL of 1% BaCl2 was put into a screw cap test tube, and then 9.95 mL of 1% H2SO4 was added. The solution was then vortexed until homogeneous and then stored in the refrigerator (22).
Antibacterial Activity Test
A petri dish containing MHA media that had been planted with Escherichia coli and Staphylococcus aureus bacteria was mixed with ethanol extract and water fraction of Pandan of leaves, chloramphenicol, and 10% DMSO. The petri dish was then incubated at 37 °C for 24 h to obtain the zone of inhibition, which was then measured with a caliper (23).
Antibacterial Activity of Ethyl Acetate Fraction and n-Hexane Fraction on Staphylococcus aureus
Preparation of Bacterial Suspension
Staphylococcus aureus bacteria were taken with a loop needle and suspended in a sterile test tube containing 0.9% NaCl solution. The bacterial suspension was then vortexed until homogeneous, and the McFarland standard was used to compare its turbidity or equivalent to 1 x 108 CFU/mL.
Preparation of MHA Media
After being poured into a sterile petri dish and solidified without any bacterial suspension, Mueller Hinton Agar was then incubated for 24 h at a temperature of 37 °C. After 24 h, the antibacterial activity was observed.
Antibacterial Acitivity Test
Antibacterial activity test was conducted using the agar well diffusion method on Staphylococcus aureus bacteria. Sterile MHA were made into wells using a sterile tube cylinder with test samples, namely 20 µL of ethyl acetate and n-Hexane fractions. The test solution was diluted using 156.25 mg of fraction dissolved using 25 mL of 10% DMSO solvent (24).
Petri dishes were then made into different concentration series, namely 20, 40, 60, and 80% v/v. This study used control petri dishes and test petri dishes. Control petri dishes consisted of media control (Mueller Hinton Agar), negative control (media + DMSO solvent), and positive control (media + clindamycin 1%). The test petri dishes consisted of Test 1 (media + 20% b/v concentration fraction), Test 2 (media + 40% b/v concentration fraction), Test 3 (media + 60% b/v concentration fraction) and Test 4 (media + 80% b/v concentration fraction) (25).
50 µL of bacteria were transferred using a micropipette to a sterile petri dish. Then, 20 mL of MHA media was added, and the media was homogenized with the bacteria in the petri dish. 20 µL of ethyl acetate fraction of Pandan leaves with concentrations of 20%, 40%, 60%, 80%, and 20 µL of n-hexane fraction of Pandan leaves with concentrations of 20%, 40%, 60%, 80% were added to each well that had been formed and then incubated for 1 x 24 h at a temperature of 37 °C. After 24 h, observations were made on forming a clear area on the MHA media. The clear area was used to indicate bacterial sensitivity to the antibacterial substance used, and then the diameter of the inhibition zone was measured in millimeters with a caliper (24).
Antibacterial Activity of n-Hexane Isolate on Escherichia coli
Preparation of Bacterial Suspension
Escherichia coli bacteria were taken, inserted, and homogenized into a sterile test tube containing NaCl solution. The bacterial suspension was then incubated at a temperature of 37 °C for 24 h, and the McFarland standard was used to compare its turbidity or equivalent to 1 x 108 CFU/mL.
Preparation of Test Concentration Series
The test concentration series was taken from the 9000 ppm stock solution by weighing 90 mg of n-hexane isolate sample in 10 mL of DMSO. The series solutions made were 500 ppm, 600 ppm, and 700 ppm. The 500 ppm test concentration series solution measured 0.16 mL of stock solution dissolved in DMSO to 3 mL. The 600 ppm test concentration series solution was carried out by measuring 0.2 mL of stock solution dissolved in DMSO to 3 mL. The 700 ppm test concentration series solution was carried out by measuring 0.23 mL of stock solution dissolved in DMSO to 3 mL.
Positive Control and Negative Control
The positive control used was the antibiotic ciprofloxacin. The positive control was made by weighing 50 mg of Ciprofloxacin powder and then dissolving it in 10 mL of DMSO. Meanwhile, the negative control used was 10% DMSO.
Antifungal Activity on Pityrosporum ovale and Preparation of Shampoo
Preparation of PDA Media
3.9 g of Potato Dextrose Agar (PDA) was dissolved into 100 mL of distilled water. Furthermore, the PDA was heated on a hot plate until boiling. NA was then sterilized by autoclaving at a temperature of 121 °C for 15 min. Then, the media was poured into a sterile petri dish and left to solidify (26).
Preparation of Positive Control
Ketoconazole shampoo 1% was used as a positive control by adding 0.1 g of ketoconazole to 10 mL of distilled water and homogenized (26).
Preparation of Control Media
The preparation of the control media only contains PDA media and fungal suspension applied to the PDA media.
Preparation of 10% Extract Test Solution
1 gram of thick extract was dissolved using 10 mL of 10% DMSO.
Preparation of Fungal Suspension
Pityrosporum ovale was cultured using PDA media and then incubated for 18-24 h at 37 °C. The colonies were then taken and put into a test tube containing 10 mL of 0.9% NaCl and homogenized. (27).
Antifungal Activity Test
Erlenmeyer from the autoclave containing PDA media was poured into a petri dish. 4 sterile Petri dishes where each dish was given a 6 mm diameter tube cylinder poured 20 mL of media and then left until the media became solid. The surface of the solidified media was poured with 100 μL of fungal suspension. The tube cylinder was then removed, and samples were added in the form of 10% extract, positive control, and 20 μL of negative control to each labeled petri dish. The petri dishes were then incubated for 24 h at 37 °C. Furthermore, the diameter of the inhibition zone in the test media was measured using a vernier caliper (mm) by looking at the clear zone indicating the fungal growth inhibition area (28).
Preparation of Anti-Dandruff Shampoo
The modified formula was based on Goel (2020) (29), as seen in Table 1. All eight shampoo formulas (F1–F8) contain 10% ethanol extract of pandan leaves as the active ingredient, supported by a consistent base of humectants, preservatives, and fragrance. The key variations lie in the surfactant composition: Sodium Lauryl Sulfate ranges from 1% to 10%, while Cocamide DEA varies from 11% to 20%, adjusting the cleansing and foaming properties across formulations. HPMC is held steady at 1.5% in most formulas, except F4 which increases it to 15% to likely influence viscosity.
Table 1. Anti-dandruff shampoo formulation of Pandan leaves ethanolic extract.
Shampo Formula (%b/v) | |||||||||
Ingredients | Function | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
Ethanol extract of Pandan leaves | Active Substance | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Sodium Lauryl Sulfate | Primary Surfactant (Detergent) | 10 | 7.75 | 3.25 | 5.5 | 10 | 10 | 1 | 1 |
Cocamide DEA | Secondary Surfactant | 11 | 13.25 | 17.75 | 15.5 | 11 | 11 | 20 | 20 |
HPMC | Base | 1.5 | 1.5 | 1.5 | 15 | 1,5 | 1.5 | 1.5 | 1,5 |
Citric Acid | Ph Increaser | qs | qs | qs | qs | qs | qs | qs | qs |
Menthol | Fragrance | 0.25 | 0.25 | 0.25 | 0.25 | 0,25 | 0.25 | 0.25 | 0.25 |
Methyl Paraben | Preservative | 0.15 | 0.15 | 0.15 | 0.15 | 0,15 | 0.15 | 0.15 | 0.15 |
Propyl Paraben | Preservative | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
Propylene Glycol | Humectant | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
Distilled Water | Solvent | Ad 50 mL | Ad 50 mL | Ad 50 mL | Ad 50 mL | Ad 50 mL | Ad 50 mL | Ad 50 mL | Ad 50 mL |
Preparation of Shampoo
All ingredients were weighed in container (A) and then mixed with HPMC in hot water, while in container (B), Sodium Lauryl Sulfate was mixed in hot water along with Cocamide DEA and propylene glycol. Then, the mixture in container (B) was mixed into container (A) until homogeneous. In container (C), methyl paraben and propyl paraben were added and dissolved with 96% ethanol and methanol. The mixture from container (C) was put into a container containing a mixture of containers (A) and (B) and stirred until homogeneous then the extract was added, and the distilled water was added to the desired volume. Physical evaluation in the form of organoleptic tests, pH, homogeneity, foam height, and viscosity were then ready to be carried out.
Optimal Formula
After the evaluation (pH, viscosity and foam height tests) of eight formulas using Design Expert software version 13.1, the optimum formula was obtained, which is said to be optimal if the formula has a desirability value close to 1.0.
Result and Discussion
Identification
Plant identification was conducted in the Laboratory of Pharmacy, Faculty of Pharmacy, Universitas Islam Sultan Agung Semarang with the following results,
- Division: Tracheophyta
- Class: Magnoliopsida
- Sub Order: Lilianae
- Order: Pandanales
- Family: Pandanaceae
- Genus: Pandanus
- Species: Pandanus amaryllifolius Roxb.
Determination Letter issued by the Pharmacy Laboratory of Sultan Agung Islamic University Semarang No: 007/B.1./SA-F/II/2024. Plant identification was carried out to obtain the appropriate and clear identity of the plants to be used in the study and to avoid errors in taking the main materials in the study. The results showed that the plant used for the study was Pandan, which was indicated by Pandanus amaryllifolius Roxb.
Extraction
Simplicia was made in several stages. In the initial stage, washing was carried out to remove other impurities attached to the simplicia, using clean running water until the leaves were completely free from dirt or foreign objects. Furthermore, shredding was carried out using a knife to help speed up the drying process. After the drying process, the simplicia was ground with a blender. The fine simplicia powder caused the surface area of the powder to increase so that the contact between the powder and the solvent was also greater so that more components contained in the simplicia could be drawn by the solvent (30).
Pandan leaves were extracted using the maceration method, where the material was soaked using a solvent in accordance with the active compound. This process can be carried out with low or even without heating (31). The maceration process requires repeated stirring or shaking to speed up the time of the solvent solution in extracting the sample (30). The maceration method is easy to do, uses simple tools, and has low damage to plant compounds. Maceration was carried out using 96% ethanol solvent. 96% ethanol was used because it has selective properties, is not a toxic substance, has good absorption power, and can separate polar and semi-polar compounds such as flavonoids and alkaloids, which have potential as antibacterial agents. In addition, 96% ethanol solvent penetration into the sample cell wall is easier than ethanol solvent with a low concentration (32).
Through the maceration method, 1,670 g of dry simplicia was soaked in 96% ethanol solvent for 3 days and stirred occasionally. The thick extract obtained was 182.5 grams with a yield of 10.9281%. In this study, the yield of the extract obtained was greater than the yield of the extract in a study by Patala (2022), namely 9.2%. This is due to the difference in solvents used. Ethanol solvent 96% can extract more chemical compounds than ethanol 70% (33). The results of the water content test of the Pandan leaves simplicia were 2.96%, and the thick extract of Pandan leaves was 5.40%. According to Utami (2017), based on Formularium Herbal Indonesia, the water content of the simplicia and extract must be ≤ 10%. This aims to avoid microbial growth in the sample. The water content results of this study indicate that the simplicia and thick extract of Pandan leaves were in accordance with the water content requirements set (34).
Phytochemical Screening
Phytochemical screening was carried out using qualitative analysis with the tube method, namely by observing color changes in the sample. The results of the phytochemical screening test of the ethanol extract of Pandan leaves are presented in Table 2.
Table 2. Phytochemical screening results.
Test Parameter | Results | Description |
Alkaloids | Orange precipitate | + |
Phenolic | Strong black | + |
Flavonoids | Orange | + |
Saponins | Foam formed | + |
Steroids / Terpenoids | Steroids: Green ring Terpenoids: Purple precipitate | + |
Phytochemical screening was conducted to determine the content of secondary metabolite compounds contained in a plant. This study used a qualitative test by observing the color reaction in the sample given the reagent. This study used a qualitative test by observing the color reaction in the sample given the reagent. In the alkaloid test, a change in the reaction causes an orange deposit. The precipitate is formed due to the addition of reagents so that a ligand replacement reaction occurs, namely nitrogen compounds in alkaloids that have free electron pairs that produce covalent bonds with K+ ions derived from potassium tetraiodobismuth and produce potassium-alkaloid complex deposits. The phenolic compound test obtained the result of the change in the color of the solution to a strong black. The change occurred because the Fe3+ ion in FeCl3 underwent a hybridization of an ion suspected to be iron (III) hexanofolate (35). This study used a qualitative test by observing the color reaction in the sample given the reagent. In the alkaloid test, a change in the reaction causes an orange deposit (36).
The identification test of saponin compounds obtained the result of the formation of foam or foam, which occurs due to the chemical composition reaction in the sample. The structure that constitutes saponins in the form of hydroxyl and carbon groups causes the compound to be soluble in water and causes foam. In addition, foam is created because glycosides form foam in water and are hydrolyzed into glucose with other compounds (37). The results of the last phytochemical compound test, namely the identification of steroid compounds, showed a green solution. The green color is produced due to the reaction between the steroid compound's OH group and the reagent in the form of Lieberman-Buchard so that a group containing green chromophores is obtained (38). The results of phytochemical screening of the ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.) showed the content of secondary metabolites in the form of flavonoids, alkaloids, phenolics, steroids, terpenoids, and saponins. This is per a study by Suryani (2018) showing that the ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.) contains secondary metabolites in the form of flavonoids, alkaloids, phenolics, steroids, terpenoids, and saponins (39).
Fractionation
Fractionation was carried out using a separating funnel. The yield of water, ethyl acetate, and n-hexane fractions is shown in Table 3.
The thick extract obtained was fractionated using a nonpolar n-hexane solvent, which breaks down leaf tissue to allow the extraction of secondary metabolites. Ethyl acetate, a semi-polar solvent with low toxicity, and ethanol-water, a polar solvent capable of dissolving most secondary metabolite compounds, were also used. Compound separation based on polarity was carried out through liquid-liquid fractionation using a separating funnel. During the fractionation process, two distinct layers formed: the upper layer contained the n-hexane fraction, and the lower layer contained the water fraction. This is due to the difference in density—n-hexane has a density of 0.4 g/mL, while water has a density of 1 g/mL—making the water fraction heavier and thus settle at the bottom. The partitioning process was repeated multiple times until the n-hexane fraction became clear, indicating that nonpolar compounds had been fully extracted into the n-hexane layer. Meanwhile, polar compounds remained in the water fraction. Subsequently, the water fraction was repartitioned with ethyl acetate, forming two new layers: the upper ethyl acetate fraction and the lower water fraction. This is because ethyl acetate has a lower density (0.66 g/mL) than water, so the water fraction remains at the bottom (40).
Table 3. Yield of water, ethyl acetate, and n-hexane fractions.
Sample | Mass | Yield (%) |
Water Fraction | 50.79 g | 32.7% |
Ethyl acetate Fraction | 52.56 g | 29.2% |
n-Hexane Fraction | 9.43 g | 23.575% |
Isolation
Isolation of Ethyl Acetate
Isolation was carried out using the TLCP method. The ethyl acetate fraction was obtained and then dropped and eluted with ethyl acetate and n-hexane eluents (3:7). After elution, observations were made under 254 nm UV light and 366 nm UV light to see the stains produced. There were 3 isolate stains produced from the ethyl acetate fraction of Pandan leaves (see Figure 1).
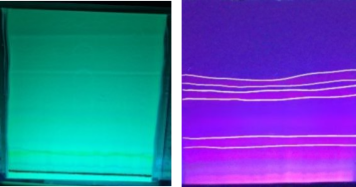
Figure 1. Isolates of ethyl acetate fraction on TLC-P.
The first ethyl acetate isolate was obtained in a quantity of 10 mg with an Rf value of 0.61. The second isolate weighed 4 mg with an Rf value of 0.50, and the third isolate weighed 10 mg with an Rf value of 0.28. Isolation using the TLC (Thin Layer Chromatography) method involves two phases: the stationary phase (adsorbent) and the mobile phase (eluent), which function to separate chemical components. These components separate based on their affinity for the stationary phase or their degree of polarity (41). TLC is a simple and rapid method frequently used in compound separation and drug analysis (42).
The ethyl acetate fraction was processed by spotting and eluting with a mixture of ethyl acetate and n-hexane in a 3:7 ratio. After elution, the TLC plates were observed under UV light at 254 nm and 366 nm, revealing three distinct spots with different Rf values. The first isolate (Rf 0.61, 10 mg) corresponded to a flavonoid compound of the quercitrin type (Rf 0.60–0.65). The second isolate (Rf 0.50, 4 mg) matched the flavonoid hyperoside (Rf 0.45–0.50). The third isolate (Rf 0.28, 10 mg) corresponded to the flavonoid rutin (Rf 0.25–0.30) (43).
Each isolate from different Rf values was tested for purity to obtain a pure isolate and indicate that the isolate obtained is a single compound. The purity test was conducted by spotting the isolate and eluting it with three eluents with different polarity indices. The 1st eluent was n-hexane and toluene (7:3), the 2nd eluent was ethyl acetate and chloroform (1.5:8.5), and the 3rd eluent was ethyl acetateand methanol (2:8). The results obtained proved that the three isolates obtained were pure compounds because there were single spots on the TLC plate. This can be interpreted as the separation pattern of the compound is good, as seen from the presence of a single stain pattern with no tail (44).
n-Hexane Isolate
The Rf values of n-Hexane isolate in stain 1 (Node 1), stain 2 (Node 2), stain 3 (Node 3), stain 4 (Node 4), stain 5 (Node 5), and stain 6 (Node 6) respectively, namely 0.80; 0.77; 0.72; 0.61; 0.5, and 0.38. Furthermore, one stain was taken, which was expected to have good antibacterial inhibition, to continue testing against Escherichia coli bacteria. The results of the TLCP of the n-Hexane isolate can be seen in Figure 2.

Figure 2. Isolates of n-hexane fraction on TLC-P. Note: (a) Node 1 Rf 0.80, (b) Node 2 Rf 0.77, (c) Node 3 Rf 0.72, (d) Node 4 Rf 0.61, (e) Node 5 Rf 0.50, and (f) Node 6 Rf 0.38.
The n-Hexane fraction from 96% ethanol extract from Pandan leaves was isolated using the TLC method to obtain pure compounds. The Preparative TLC method has a more efficient separation time. The resulting stain is easy to observe and easy to do. Isolation was carried out using the eluent n-Hexane: Ethyl Acetate: Methanol (7:3:1). The selection of eluents was carried out using the TLC method, considering the best optimization and separation results. Ethyl acetate eluent was used because it is a semi-polar solvent, so the visible stain can come from nonpolar or polar compounds. The isolation process begins by preparing the n-hexane fraction, then dissolving it with 1ml of chloroform and 1ml of methanol. The selection of fraction solvents with chloroform and methanol is because chloroform is nonpolar and methanol is polar, so both can dissolve the n-hexane fraction. The stains obtained on the TLC plate were observed under UV lamps at 256 nm and 366 nm with the aim that the stains were visible. After that, the Rf value on the visible stain was then calculated. The Rf value is calculated as the distance traveled by the component divided by the distance traveled by the eluent (mobile phase) for each compound. The Rf values obtained from the stains on the TLC plate of n-hexane isolate are classified as good because it has a value of 0.2 to 0.8. The isolate used for antibacterial testing was stain 1 because stain 1 is a target compound with a good Rf value, and it is suspected that stain 1 is an alkaloid compound because it has an Rf value of 0.8. Previous research by Maziyah (2019) showed that the n-hexane fraction of Pandan leaves contains alkaloid, saponin, and flavonoid compounds, which can inhibit bacterial growth (45). The results of n-hexane isolation were tested for antibacterial activity in bacteria Escherichia coli.
Isolate Purity Test
Purity Test of Ethyl Acetate Isolate and n-Hexane Isolate
This study conducted a purity test using three eluents for ethyl acetate fraction with different polarity indexes. In the 1st eluent, namely n-hexane and toluene (7:3), the 2nd eluent was ethyl acetate and chloroform (1.5:8.5), and the 3rd eluent was ethyl acetate and methanol (2:8). The results showed that there was 1 stain that appeared on the TLC plate in each eluent used. These results show that the isolate obtained is a pure compound, as presented in Figure 3. The purity test of Pandan leaf isolates was conducted using the TLC method. The eluents used were n-hexane:toluene (7:3), n-hexane:ethyl acetate (7:3), and chloroform:methanol (8:2) with Rf values of 0.25, 0.72, and 0.45 respectively. The purity test result of the n-hexane isolate can be seen in Figure 4.

Figure 3. Purity test results of ethyl acetate isolates. Note: black arrow points to a single node.

Figure 4. Purity test result of n-hexane isolates.
The purity test of the n-hexane isolate was conducted using the TLC method with three different eluent ratios to determine the most effective eluent based on polarity. TLC is a simple and relatively fast method for assessing sample purity. It is particularly practical for small-scale analyses, as it requires only a small amount of material. The three eluent ratios used were n-hexane:toluene (7:3), n-hexane:ethyl acetate (7:3), and chloroform:methanol (8:2). The purpose of the purity test was to confirm whether the compound in stain 1 was a single compound. With the first eluent (n-hexane:toluene, 7:3), a single spot was observed with an Rf value of 0.25. The second eluent (n-hexane:ethyl acetate, 7:3) also produced a single spot with an Rf value of 0.72. The third eluent (chloroform:methanol, 8:2) yielded a single spot with an Rf value of 0.45. Eluent selection is influenced by the polarity or solubility of the compounds in the n-hexane isolate. Variations in Rf values can result from several factors, including the type, properties, and size of the TLC plate, the direction of mobile phase flow, the composition of the mobile phase, and the sample preparation method.
Antibacterial and Antifungal Activity Test
The results of the antibacterial and antifungal activity tests of the ethanol extract, water, ethyl acetate, and n-hexane fractions, as well as the ethyl acetate and n-hexane isolates, are presented in Table 4.
Table 4. Results of the antibacterial and antifungal activity tests.
Sample | Concentration | Staphylococcus aureus (mm) | Escherichia coli (mm) | Pityrosporum ovale (mm) | Category |
Ethanol Extract 96% | 600 ppm | - | 10.8 | - | Intermediate |
800 ppm | - | 15.8 | - | Intermediate | |
10% | - | - | 6.29 | Intermediate | |
Water Fraction | 70% | 8.76 | 10.36 | - | Intermediate |
80% | 9.8 | 10.13 | - | Intermediate | |
90% | 8.56 | 10.86 | - | Intermediate | |
100% | 9.96 | 11.53 | - | Intermediate | |
Ethyl Acetate Fraction | 20% | 0 | - | - | - |
40% | 0 | - | - | - | |
60% | 0 | - | - | - | |
80% | 0 | - | - | - | |
600 ppm | - | 0 | - | - | |
800 ppm | - | 0 | - | - | |
n-Hexane Fraction | 20% | 2.4067 | - | - | Resistant |
40% | 6.613 | - | - | Resistant | |
60% | 6.0467 | - | - | Resistant | |
80% | 3.78 | - | - | Resistant | |
Ethyl Acetate Isolate | 600 ppm | - | 0 | - | - |
800 ppm | - | 0 | - | - | |
n-Hexane Isolate | 500 ppm | - | 13.9 | - | Intermediate |
600 ppm | - | 15.5 | - | Intermediate | |
800 ppm | - | 19.6 | - | Strong |
The results of the antibacterial activity test on the ethanol extract, ethyl acetate fraction, and n-hexane isolate at concentrations of 600 ppm and 800 ppm showed that only the ethanol extract, water fraction, and n-hexane isolate of pandan leaves exhibited zones of inhibition, indicating antibacterial activity against Escherichia coli. The ethanol extract at 600 ppm produced an average inhibition zone of 10.8 mm, categorized as intermediate, while at 800 ppm it produced an average inhibition zone of 15.8 mm, also within the intermediate category, based on CLSI standards (2020). This is per a study by Rosidah et al. in 2021 that at 600 ppm and 800 ppm, ethanol extract of Pandan leaves can inhibit gram-negative bacteria with moderate and strong categories (20). Meanwhile, the ethyl acetate fraction and ethyl acetate isolate did not show antibacterial activity with no formation of the inhibition zone, which means that in the same concentration as the ethanolic extract of Pandan leaves, it cannot inhibit the growth of Escherichia coli bacteria. Alkaloid, phenolic and flavonoid compounds that can inhibit bacteria were identified in the ethyl acetate fraction from the results of the phytochemical screening test, but in reality, no inhibition zone was formed in the ethyl acetate fraction. This is because the compound levels are unknown, so it cannot be ascertained that it has antibacterial activity. As explained by previous studies, the levels of a compound are directly proportional to the pharmacological activity possessed by a sample (46). Isolates obtained from 3 different Rf values are included in the flavonoid group compounds such as quercitrin, hyperoside, and rutin (43). Based on previous studies, flavonoid compounds can work as antibacterials by disrupting the synthesis of bacterial cell macromolecules, causing damage to the permeability of bacterial cell walls (47). However, the three isolates obtained in this study did not show an inhibition zone around the agar well diffusion holes. This is because the amount of concentrate used in extracting flavonoid compounds that act as antibacterial is lower, so it cannot inhibit the growth of Escherichia coli bacteria. Suppose the sample concentration used in the antibacterial activity test is greater. In that case, the active substance dissolved in the sample is also greater, so the inhibition zone formed is greater (46).
The n-hexane fraction sample of pandan leaves formed an inhibition zone against the growth of Staphylococcus aureus. The inhibition zones varied with concentration: 2.41 mm at 20%, 6.61 mm at 40%, 6.05 mm at 60%, and 3.78 mm at 80%. In contrast, the ethyl acetate fraction showed no inhibition zone against S. aureus. In a study by Setiyanto R. et al. (2024), antibacterial activity testing was also conducted using the ethyl acetate fraction of pandan leaves against E. coli at 10%, 20%, and 30%. The results showed no inhibition zones, indicating that the ethyl acetate fraction lacked antibacterial activity. The study used the disc diffusion method and confirmed that the compound content in the ethyl acetate fraction had no antibacterial effect against E. coli (48). According to CLSI standards, antibacterial activity is categorized based on inhibition zone diameter: <14 mm indicates weak or resistant activity, 15–19 mm indicates moderate or intermediate activity, and >20 mm indicates strong or susceptible activity (49). Based on this classification, the n-hexane fraction falls into the resistant category, as shown by the relatively small inhibition zones observed. The lack of antibacterial activity in the ethyl acetate fraction is likely due to the structure of S. aureus, whose cell wall is composed of peptidoglycan, polysaccharides, proteins, and low-fat content. This structure allows nonpolar compounds to penetrate more easily, which may explain why the semi-polar compounds present in the ethyl acetate fraction were ineffective (17).
The n-hexane fraction at concentrations of 40% and 60% produced larger average inhibition zone diameters, while at 20% and 80%, the inhibition zones were smaller. This aligns with a study by (50), which found that several factors, including the test sample's concentration and the bacterial suspension's turbidity, can influence antibacterial activity. A higher test sample concentration can affect the stock solution's consistency, potentially reducing the number of active compounds that diffuse into the medium. For the water fraction sample at concentrations of 70%, 80%, 90%, and 100%, the inhibition zones against Escherichia coli were 10.36 mm, 10.13 mm, 10.86 mm, and 11.53 mm, respectively—indicating that the water fraction possesses antibacterial activity. Meanwhile, the water fraction produced inhibition zones of 8.76 mm, 9.8 mm, 8.56 mm, and 9.96 mm against Staphylococcus aureus. Based on CLSI (2020) guidelines (49), these values classify the activity as resistant (≤12 mm). An inhibition zone of 13–17 mm is considered intermediate, and ≥18 mm is categorized as sensitive. Among the three fractions, the water fraction was the most effective in inhibiting the growth of both Staphylococcus aureus and Escherichia coli.
The inhibition of Escherichia coli by the n-hexane isolate of Pandan leaves was tested at concentrations of 500 ppm, 600 ppm, and 700 ppm. The ability of this isolate to inhibit E. coli growth is attributed to the presence of alkaloid compounds. Alkaloids contain nitrogen groups that act as antibacterial agents. They work by disrupting the bacterial cell wall, damaging the peptidoglycan structure, and preventing the proper formation of the cell wall (51). The inhibition zones observed were 19.6 mm at 700 ppm, 15.5 mm at 600 ppm, and 13.9 mm at 500 ppm. These results indicate that higher concentrations result in stronger antibacterial activity. Therefore, the n-hexane isolate demonstrates effective antibacterial activity against Escherichia coli. Additionally, a 96% ethanol extract showed an inhibition zone of 6.29 mm. Based on standard classifications, antifungal activity is grouped into four categories: an inhibition zone of <5 mm is considered weak or resistant, 6–10 mm is moderate or intermediate, 11–20 mm is strong, and >20 mm is very strong (9). Thus, the ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.) falls into the moderate antifungal activity category. This antifungal activity is likely due to the presence of secondary metabolites, such as flavonoids and alkaloids, which are known to inhibit fungal growth.
Physical Properties Test of Shampoo from Ethanol Extract of Pandan leaves (Pandanus amaryllifolius Roxb.) with Surfactants
Organoleptic Test
The organoleptic test was conducted by visually observing the shampoo from ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.), especially in terms of shape, color, and smell based on appropriate parameters such as semi-solid (thick) shape, dark green in color, with a distinctive odor of Pandan leaves (Pandanus amaryllifolius Roxb.) (52). The results of observations on eight formulas with a combination of surfactants in the form of sodium lauryl sulfate and cocamide DEA did not significantly affect the organoleptic test. In formulas I to VIII, there was no difference in shape, color, and smell. In the organoleptic test, the shape or texture of the shampoo was influenced by the shampoo base used, namely HPMC, where the higher the concentration of HPMC, the thicker the shampoo will be. Based on the organoleptic test, formulas I to VIII have met the requirements because they have the same characteristics of shape, color, and smell (53).
Homogeneity Test
The homogeneity test was conducted by observing whether coarse grains were in the shampoo from the ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.). The results of the homogeneity analysis on 8 formulas showed that there were no coarse grains in the shampoo, so it can be stated that the 8 formulas are homogeneous. This indicates that all the ingredients used have been dispersed properly and correctly (53). Ethanol extracts do not always affect the homogeneity of shampoo preparations. Still, the base used can affect the homogeneity because inappropriate bases can cause the ingredients to not mix homogeneously (54).
pH Test, Viscosity Test, and Foam Height Test
The pH test was conducted on eight formulas to ensure compatibility between the shampoo's and skin's pH, ensuring safety and comfort (see Table 5). The ideal pH range for shampoo is 5.5–6. If the pH is too acidic, it can irritate the scalp; if it's too alkaline, it can cause dryness. The results showed that the pH of the shampoo formulas exceeded the acceptable range (55). One contributing factor is the concentration of sodium lauryl sulfate, which affects the preparation's pH. Higher concentrations of sodium lauryl sulfate can raise the pH, as it has basic properties with a pH of 7.5–8.5 (56). Other factors influencing pH include storage conditions, temperature, humidity, and light exposure, which can affect material stability (57). A viscosity test was also performed to determine the thickness of each formula, which influences the shampoo's effectiveness and user experience. Testing was done using a Brookfield viscometer with a spindle size of 63 at 20 RPM. All eight formulas met the required viscosity range of 400–4000 cps. In general, higher concentrations resulted in thicker shampoo preparations. Foam height testing was conducted to ensure the amount of foam produced was appropriate. Ideal foam height for shampoo ranges from 1.3 to 22 cm. The results showed that all eight formulas met this requirement as presented in Table 5.
Table 5. Viscosity, pH, and foam height of Pandan shampoo formula.
Formula | pH | Viscosity (cps) | Foam height (cm) |
F1 | 7.69 ± 0 | 1170 ± 10.39 | 8.83 ± 1.04 |
F2 | 8.19 ± 0 | 529.9 ± 99.73 | 15.66 ± 0.57 |
F3 | 8.24 ± 0 | 1911.66 ± 508.95 | 9.16 ± 0.76 |
F4 | 8.01 ± 0.01 | 987.86 ± 48.98 | 9.56 ± 0.92 |
F5 | 7.8 ± 0 | 903.8 ± 12.49 | 12.33 ± 0.57 |
F6 | 8.04 ± 0 | 593.9 ± 31.74 | 11.5 ± 0.5 |
F7 | 8.52 ± 0.02 | 901.9 ± 217.78 | 10.66 ± 0.57 |
F8 | 8.83 ± 0.01 | 839.8 ± 98.22 | 10 ± 1 |
Formula optimization was carried out using the Simplex Lattice Design (SLD) method to determine the ideal concentration of various ingredients and obtain a formula with the desired physical properties. The SLD method is fast and practical, avoiding the need for trial-and-error formulation. It also allows optimizing two or more ingredient combinations to achieve the best possible formula (58). The optimization process produced eight formulas combining a primary surfactant (sodium lauryl sulfate) and a secondary surfactant (cocamide DEA). These formulas were evaluated through physical tests, including pH, viscosity, and foam height. Once the eight formulas were prepared, shampoo samples were made according to the SLD outputs. Physical tests were then conducted, and the results were fed back into the SLD for analysis to determine the optimum formula. The optimum pH and foam height were selected based on in-range response criteria, while viscosity was optimized using a target response goal. The best solution was identified with a desirability value close to 1.0, indicating optimal formulation. The ideal combination was found to be 0.235 parts sodium lauryl sulfate and 0.764 parts cocamide DEA. A desirability value close to 1.0 indicates a more optimal product outcome (9). The anti-dandruff shampoo using ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.) was formulated based on Simplex Lattice Design analysis to determine the optimal proportions of sodium lauryl sulfate and cocamide DEA as surfactants (see Table 6).
Table 6. Results of phyical optimization and evaluation of ethanol extract shampoo from pandan leaves.
Physical Properties | Mean ± SD | Predicted Value |
pH | 5.71 ± 0.07 | 8.40 |
Foam height (cm) | 2.56 ± 0.40 | 10.72 |
Viscosity (cps) | 899.9 ± 207.62 | 1793.44 |
The results of the physical evaluation test, conducted in three replications, were then compared with the predictions from the Simplex Lattice Design (SLD). The physical test results of the anti-dandruff shampoo formulated with ethanol extract of Pandan leaves (Pandanus amaryllifolius Roxb.) did not show significant differences from the SLD predictions. This indicates that using the SLD method effectively identified the most ideal and appropriate formula for the anti-dandruff shampoo, using a valid and reliable combination of sodium lauryl sulfate and cocamide DEA (13).
Conclusion
The ethanol extract of pandan wangi leaves (Pandanus amaryllifolius Roxb.) shows effective antibacterial activity at 800 ppm against E. coli and antifungal activity against Pityrosporum ovale at a concentration of 10%. Among the water, ethyl acetate, and n-hexane fractions, the water fraction was the most effective in inhibiting E. coli and S. aureus. For the isolates, the n-hexane isolate demonstrated antibacterial activity against E. coli, indicating that the n-hexane isolate is the most effective, classified as strong. Against S. aureus, the water fraction was the most effective, falling into the moderate category. The ethanol extract also showed moderate antifungal activity against Pityrosporum ovale.
In the shampoo formulation, the optimal surfactant ratio was 0.764 for cocamide DEA and 0.235 for sodium lauryl sulfate, equivalent to 17.876% and 3.124%, respectively. The physical evaluation results showed a pH of 5.71, foam height of 2.56 cm, and viscosity of 899.9 cps—all meeting the required standards.
Abbreviations
TLC = Thin Layer Chromatography, Cocamide DEA = Cocamide Diethanolamine, LAF =, HPMC = Hydroxypropyl Methylcellulose, PDA = Potato Dextrose Agar, DMSO = Dimethyl Sulfoxide, UV = Ultraviolet Rays, MHA = Mueller Hinton Agar, NA = Nutrient Agar, CLSI = Clinical and Laboratory Standards Institute, SLD = Simplex Lattice Design.
Declarations
Ethics Statement
Not applicable.
Data Availability
All data in this study have been published in the manuscript, but if readers need more information about this research/explanation of the data that has been presented, then they can contact the corresponding author via email wijayanti@unissula.ac.id.
Funding Information
This work was funded by LPPM Universitas Islam Sultan Agung.
Conflict of Interest
The authors declare no conflicting interest.
References
- Aisyah LS, Jasmansyah J, Purbaya S, Resnawati T. Isolation and Antibacterial Activity of Phenol Compounds of Ethyl Acetic Extract of Red Zinger (Zingiber officinale Roscoe var. sunti). J Kartika Kim. 2019;2(1):44–50.
- Gherardi G. Staphylococcus aureus Infection: Pathogenesis and Antimicrobial Resistance. Int J Mol Sci. 2023;24(9).
- Apriliantisyah W, Haidir I, Rasfayanah, Sodiqah Y, M. Said MF. Daya Hambat Ekstrak Kunyit (Curcucma domestica Val) Terhadap bakteri Staphylococcus aureus dan Escherichia coli. Fakumi Med J J Mhs Kedokt. 2022;2(10):694–703.
- Laelasari E, Musfiroh I. Review Article : Potential of Herbal Plants Against Pityrosporum ovale Fungus Causes of Dandruff. Indones J Biol Pharm. 2022;2(3):152–8.
- Malonda TC, Yamlean PVY, Citraningtyas G. Formulasi Sediaan Sampo Antiketombe Ekstrak Daun Pacar Air ( Impatiens balsamina L .) dan Uji Aktivitasnya Terhadap Jamur Candida Albicans Atcc 10231 Secara In Vitro. 2017;6(4).
- Furqon M, Martalena Silitonga E, Tarigan FLB. Formulasi Sediaan Sampo Antiketombe Esktrak Daun Alpukat (Persea americana Mill) dan Uji Aktivitasnya Terhadap Jamur Pityrosporum ovale. J TEKESNOS. 2021;3(2):330–40.
- Sudigdoadi STRAPIB. Mekanisme Timbulnya Resistensi Antibiotik Pada Infeksi Bakteri. Fak Kedokt Univeritas Padjadjaran. 2015;1–14.
- Nurdianti L. Pengembangan Formulasi Sediaan Gel Rambut Antiketombe Ekstrak Daun Pandan Wangi (Pandanus amaryllifolius Roxb.) dengan Menggunakan Viscolam Sebagai Gelling Agent dan Uji Aktivitasnya Terhadap Jamur Pityrosporum ovale. J Kesehat Bakti Tunas Husada J Ilmu-ilmu Keperawatan, Anal Kesehat dan Farm. 2018;17(2):456.
- Sinaga A, Siregar S, Rizky VA, Topia R. Antifungal effectiveness test fragrant leaf ethanol extract (Pandanus Amaryllifolium Robx) against fungus Pityrosporum Ovale in vitro. ITEGAM- J Eng Technol Ind Appl. 2021;7(31).
- Lomthong T, Chorum M, Samaimai S, Thongpoem P. Antioxidant and antibacterial activities of Pandanus amaryllifolius Roxb. (Pandanaceae) prop roots and its application for a novel bacterial cellulose (Nata) fermentation by enzymatic hydrolysis. J Appl Biol Biotechnol. 2022 Jun 1;147–52.
- Wahyuni DK, Nuha GA, Atere TG, Kharisma VD, Tari VS, Rahmawati CT, et al. Antimicrobial potentials of Pandanus amaryllifolius Roxb.: Phytochemical profiling, antioxidant, and molecular docking studies. de Oliveira MS, editor. PLoS One. 2024 Aug 14;19(8):e0305348.
- Dumaoal OSR, Alaras LB, Dahilan, Sarah KG, Depadua AA, Pulmones CJG. In Vitro Activity of Pandan (Pandanus amaryllifolius) Leaves Crude Extract Against Selected Bacterial Isolates. JPAIR Multidiscip Res. 2010 Jan 25;4(1):102–24.
- Lestari DA, Juliantoni Y, Hasina R. Optimasi formula sampo ekstrak daun pacar air (Impatiens balsamina L.) dengan kombinasi natrium lauril sulfat dan cocamide DEA. Sasambo J Pharm. 2021;2(1):23–31.
- Kayadoe V, Fadli M, Hasim R, Tomasoa M. Ekstrak daun pandan. Molekul. 2015;10:88–96.
- Wahyuni I. Uji Daya Hambat Ekstrak Daun Pandan Wangi (Pandanus Amaryllifolius Roxb) Terhadap Bakteri Eschericia Coli. Jimvet. 2018;15(2):1–8.
- Rahmasiahi, Hadiq S, Yulianti T. Skrining Fitokimia Ekstrak Metanol Daun Pandan (Pandanus amarillyfolius Roxb). J Pharm Sci Herb Technol. 2023;1(1):32–9.
- Mardiyaningsih A, Aini R. Pengembangan Potensi Ekstrak Daun Pandan (Pandanus amaryllifolius Roxb) Sebagai Agen Antibakteri. Pharmaciana. 2014 Nov 1;4(2).
- Angelina M. Uji Aktivitas Antibakteri Ekstrak Etanol Daun Kemangi (Ocimum sanctum L.) Terhadap Pertumbuhan Bakteri Escherichia coli. J Novem Med Farm. 2023;2(2):60–6.
- Berlian Z, Fatiqin A, Agustina E. Penggunaan Perasan Jeruk Nipis (Citrus aurantifolia) dalam Menghambat Bakteri Escherichia coli pada Bahan Pangan. Bioilmi J Pendidik. 2016;2(1).
- Rosidah. R, Lili W, Hamdani H, Sopiah S. Antimicrobial activity of pandanus leaves extract to against Aeromonas hydrophila which attacked catfish. Int J Fish Aquat Stud. 2021;9(1):14–7.
- Mardiyaningsih A, Aini R. Pengembangan Potensi Ekstrak Daun Pandan (Pandanus amaryllifolius Roxb) sebagai Agen Antibakteri. Pharmaciana. 2014;4(2):185–92.
- Rosmania R, Yanti F. Perhitungan jumlah bakteri di Laboratorium Mikrobiologi menggunakan pengembangan metode Spektrofotometri. J Penelit Sains. 2020;22(2):76.
- Arfa Yanti N, Ambardini S, Ode Leni Marlina W, Dwi Cahyanti. Aktivitas Antibakteri Kombucha Daun Sirsak (Annona muricata L.) Dengan Konsentrasi Gula Berbeda (Antibacterial Activity of Soursoup Leaves Kombucha (Annona muricata L.) With Different Sugar Concentration). J Biol FMIPA Univ Halu Oleo. 2020;8(2):35–40.
- Nurhayati LS, Yahdiyani N, Hidayatulloh A. Perbandingan Pengujian Aktivitas Antibakteri Starter Yogurt dengan Metode Difusi Sumuran dan Metode Difusi Cakram. J Teknol Has Peternak. 2020;1(2):41.
- Juariah S, Wiranda J, Sepryani H. Uji Efektivitas Ekstrak Daun Pandan Wangi (Pandanus amaryllifolius Roxb) terhadap Pertumbuhan Bakteri Streptococcus mutans. J Indones Med Lab Sci. 2022;3(1):89–96.
- Yusuf M, Alyidrus R, Irianti W, Farid N. Uji Aktivitas Antifungi Ekstrak Etanol Kulit Nanas (Ananas comosus (L.) Merr) Terhadap Pertumbuhan Pityrosporum ovale dan Candida albicans Penyebab Ketombe. Media Kesehat Politek Kesehat Makassar. 2020;15(2):311.
- Taufiqurrahman M, Pijaryani I. Uji Mutu Fisik Formula Sampo Ekstrak Kulit Markisa (Passiflora edulis) Sebagai Antiketombe. J Ilmu Kefarmasian. 2023;4(1):224–8.
- Gantoro B, Amelia C, Sholikhah NA. Pengaruh Pemberian Rebusan Daun Pandan Wangi (Pandanus amaryllifolius Roxb) Terhadap Penurunan Glukosa Darah Pada Mencit (Mus musculus). Zo Kedokt Progr Stud Pendidik Dr Univ Batam. 2022;12(2):87–96.
- Goel R, Bhardwaj S, Bana S. Pharmaceutical excipients. In: Dosage Forms, Formulation Developments and Regulations. Elsevier; 2024. p. 311–48.
- Lady Yunita Handoyo D, Pranoto ME. Pengaruh Variasi Suhu Pengeringan Terhadap Pembuatan Simplisia Daun Mimba (Azadirachta Indica). J Farm Tinctura. 2020;1(2):45–54.
- Chairunnisa S, Wartini NM, Suhendra L. Effect of Temperature and Maseration Time on Characteristics of Bidara Leaf Extract (Ziziphus mauritiana L.) as Saponin Source. J Rekayasa dan Manaj Agroindustri. 2019;7(4):551–60.
- Wendersteyt NV, Wewengkang DS, Abdullah SS. Uji Aktivitas Antimikroba dari Ekstrak dan Fraksi Ascidian Herdmania momus dari Perairan Pulau Bangka Likupang terhadap Pertumbuhan Mikroba Staphylococcus aureus, Salmonella typhimurium, dan Candida albicans. Pharmacon. 2021;10(1):706.
- Patala R, Mandang MA, Tandi J. Uji efek ekstrak etanol daun pandan wangi terhadap histopatologi ginjal tikus putih diinduksi streptozotocin. Farmakol J Farm. 2022;XIX(1):67–77.
- Putri TUA, Sumekar DW. Uji efektivitas daun salam (Syzygium polyanthum ) sebagai antihipertensi pada tikus galur wistar. “ Uji Ef daun salam (Syzygium polyanthum ) sebagai antihipertensi pada tikus galur wistar Major 6(1), 77–81.” 2017;6(1):77–81.
- Habibi AI, Firmansyah RA, Setyawati SM. Skrining fitokimia ekstrak n-Heksan korteks batang salam (Syzygium polyanthum). Indones J Chem Sci. 2018;7(1):1–4.
- Munadi R, Arifin L. Identifikasi Senyawa Metabolit Sekunder dan Uji Aktivitas Antioksidan Ekstrak Daun Jahe Putih (Zingiber officinale Rosc. var. officinarum). Spin. 2022;4(2):163–74.
- Agustina W, Nurhamidah, Handayani D. Skrining Fitokimia dan Aktivitas Antioksidan Beberapa Fraksi Dari Kulit Batang Jarak (Ricinus communis L.). J Pendidik dan Ilmu Kim. 2017;1(2):117–22.
- Sahriawati S, Sumarlin S, Wahyuni S. Validasi Metode dan Penetapan Kadar Kolesterol Ayam Broiler dengan Metode Lieberman- Burchard. Lutjanus. 2020;24(2):31–40.
- Suryani CL, Murti STC, Ardiyan A, Setyowati A. Aktivitas Antioksidan Ekstrak Etanol Daun Pandan (Pandanus amaryllifolius) dan Fraksi-Fraksinya. Agritech. 2018;37(3):271.
- Fauziah ED, Bialangi N, Musa WJA. Isolasi dan Karakterisasi Senyawa Aktif Terhadap Mortalitas Kutu Beras dari Ekstrak Etil Asetat Rimpang Jeringau ( A corus calammus L .). J Entropi. 2017;12(1):25–32.
- Alen Y, Agresa FL, Yuliandra Y. Analisis Kromatografi Lapis Tipis (KLT) dan Aktivitas Antihiperurisemia Ekstrak Rebung Schizostachyum brachycladum Kurz (Kurz) pada Mencit Putih Jantan. J Sains Farm Klin. 2017;3(2):146.
- Mulkin A, Maarisit W, Pareta D, Palandi RR. Identifikasi Bahan Kimia Obat (BKO) Glibenklamid Pada Jamu Antidiabetes Dengan Menggunakan Metode Kromatografi Lapis Tipis (KLT) Dan Spektrofotodensitometri. Biofarmasetikal Trop. 2020;3(2):48–53.
- Sri Sulasmi E, Adi Nugraha L, Sapta Sari M, Juriusan Biologi S, Negeri Malang Jalan Semarang Malang No U. Skrining Fitokimia Dan Analisis Kromotografi Lapis Tipis Dari Senyawa Aktif Kalakai (Stenochlaena palustris (Burm.F) Beddome) Di Taman Nasional Baluran. Pros Semin Nas VI Hayati 2018. 2018;VI(September):1–9.
- Bialangi N, Idris RR, La Kilo A, Kilo AK. Isolasi Dan Identifikasi Senyawa Metabolit Sekunder Ekstrak Etil Asetat Daun Sambiloto. Jambura J Chem. 2022;4(1):25–32.
- Maziyah I. Uji Aktivitas Antibakteri Fraksi n-Heksan dan Etanol Ekstrak Daun Pandan Wangi (Pandanus amaryllifolius Roxb.) terhadap Bakteri Staphylococcus aureus. 2019;(November):1–23.
- Mastra N. Perbedaan Zona Hambat Pertumbuhan Staphylococcus aureus pada Berbagai Konsentrasi Rebusan Daun Salam (Syzygium polyanthum) secara In Vitro. Meditory J Med Lab. 2018;5(2):92–100.
- Fiana FM, Kiromah NZW, Purwanti E. Aktivitas Antibakteri Ekstrak Etanol Daun Sukun (Artocarpus altilis) Terhadap Bakteri Staphylococcus aureus Dan Escherichia coli. Pharmacon J Farm Indones. 2020;10–20.
- Setiyanto R, Suhesti I, Utami AD. Antibacterial and antifungi activity of pandan wangi (Pandanus amaryllifolius Roxb)leaf ethanolic extract and its n-heksan and etil acetate fraction. J Ilm Farm. 2024 Jul 31;20(1):156–68.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Wayne PA: Clinical and Laboratory Standards Institute; 2018.
- Pehino A, Fatimawali F, Suoth EJ. Uji Aktivitas Antibakteri Ekstrak Biji Buah Duku (Lansium domesticum) terhadap Bakteri Staphylococcus aureus dan Escherichia coli. Pharmacon. 2021 May 17;10(2):818.
- Ariana diah. Uji Antibakteri Perasan Daun Pandan Wangi (Pandanus amaryllifolius Roxb) terhadap Shigella dysenteriae. Surabaya J Muhamadiyah Med Lab Technol. 2018;1(1):67–72.
- Danayasa IK, Yudianti Mendra NN, Sunadi Putra IMA. Formulasi Sediaan Shampo Kombinasi Ekstrak Daun Pandan Wangi (Pandanus amaryllifolius Roxb) dan Daun Seledri (Apium graveolens L.) sebagai Antiketombe terhadap Jamur Candida albicans. Usadha. 2023;2(4):7–13.
- Salsabila HG. Optimasi Konsentrasi Basis HPMC Sediaan Sampo Antiketombe Ekstrak Daun Belimbing Wuluh (Averrhoa bilimbi L.) Kombinasi Ekstrak Daun Pandan Wangi (Pandanus amaryllifolius Roxb). Proceeding Mulawarman Pharm Conf. 2021;(April 2021):135–8.
- Sugiyono, Zein HS, Murrukmihadi M. Pengaruh Konsentrasi HPMC sebagai Gelling Agent terhadap Sifat Fisik dan Stabilitas Gel Ekstrak Etanol Daun Ubi Jalar (Ipomoea batatas L .). Media Farm Indones. 2014;9(2):792–9.
- Gubitosa J, Rizzi V, Fini P, Cosma P. Hair Care Cosmetics: From Traditional Shampoo to Solid Clay and Herbal Shampoo, A Review. Cosmetics. 2019 Feb 19;6(1):13.
- Nareswari TL, Nurjannah O, Sari LMNI, Syafitri E. Pengaruh Variasi Surfaktan terhadap Sifat Fisik Sampo Berbasis Minyak Serai Wangi (Cymbopogon nardus (L.) Rendle) dan Ekstrak Lidah Buaya (Aloe vera). J Farm Malahayati. 2023 Jan 3;5(2):155–64.
- Eryaputri NRAS, Triannisa S, Damayanti AF, Za’ani AJ, Eggy MF, Farhan M, et al. Effect of the Addition Variations Cocamide Diethanolamine on Physical Characteristics Preparation of Citronella Oil Shampoo. Indones J Chem Sci. 2023;12(2):120–9.
- Gumbara YT, Murrukmihadi M, Mulyani S. Optimasi Formula Sediaan Lipstik Ekstrak Etanolik Umbi Ubi Jalar Ungu (Ipomea batatas L.) dengan Kombinasi Basis Carnaubawa Wax dan Paraffin Wax Menggunakan Metode SLD (Simplex Lattice Design). Maj Farm. 2015;11(3):336.

 ETFLIN
Notification
ETFLIN
Notification






