Advances in Terpenoid Biosynthesis: Chemical Diversity and Emerging Industrial Applications
by Harshal Shivaji Patil ★ , Ashwini Sanjay Baviskar ★ , Jaysing Mahavirsing Dinore, Ajeet Appasaheb Yelwande
Academic editor: Bethsebie Lalduhsaki Sailo
Sciences of Phytochemistry 4(2): 62-75 (2025); https://doi.org/10.58920/sciphy0402310
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
04 Mar 2025
02 Jul 2025
21 Aug 2025
03 Sep 2025
Abstract: Terpenoids are the largest and most chemically diverse class of natural products, essential for plant functions such as growth regulation, defense, and ecological interactions. Their extensive chemical variety and functional versatility have also sparked significant industrial interest across many sectors. This review highlights recent progress in terpenoid biosynthesis, especially focusing on the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways, which are the main routes for isoprenoid precursor production. It explores the enzymatic processes that create complex terpenoid skeletons, including detailed cyclization and rearrangement steps carried out by terpene synthases and modifying enzymes. Advances in metabolic engineering and synthetic biology now allow the reconstruction and improvement of terpenoid pathways in microbial and plant systems, greatly increasing production yields. The use of bioinformatics and systems biology tools has further supported pathway discovery, enzyme analysis, and strain development. Beyond their traditional uses in drugs, nutraceuticals, flavors, and fragrances, terpenoids are also promising for biofuels and renewable materials, emphasizing their industrial value. This review addresses challenges such as pathway complexity, precursor supply, and regulatory control, and suggests strategic directions for future research. Overall, these insights reinforce the importance of terpenoids as key targets for sustainable biotech innovations.
Keywords: Terpenoid biosynthesisIsoprenoid metabolismMevalonate (MVA) pathwayTerpene synthasesIndustrial applications of terpenoids
Introduction
Isoprenoids are the most ancient and diverse class of “natural products” having over 80,000 individual structures with an eclectic array of carbon skeletons and functional groups (1-4). Isoprenoids exhibiting a vast array of chemical structures are mainly found in plants. It ranges from universal primary metabolites, such as sterols (essential components of biomembranes), carotenoids and chlorophyll (photosynthetic pigments), ubiquinones, vitamins, and hormones (involved in plant defense and communication), to secondary metabolites including terpenoids (5-7). The word “terpene” originated from turpentine, which is a semi-fluid oleoresin extracted from coniferous trees (8). Terpenoids are the modified derivatives of the terpene skeleton, which are formed by chain elongation and cyclization with the inclusion of functional groups on the hydrocarbon skeleton. Terpenoids are primarily involved in plant defense mechanisms, adaptation, reproduction, and chemical signaling, ensuring survival under various conditions (7, 9). Terpenoids possess several potent pharmacological activities, including antimicrobial, anti-inflammatory, anti-allergic, anti-parasitic, anticancer, antidiabetic, insecticidal, and antimalarial effects (10-12). Two such commercially successful anticancer drugs, Taxol and artemisinin, are terpene derivatives from Taxus brevifolia and Artemisia annua, respectively (13, 14). Recently, terpenoids have emerged as a potential biofuel due to their chain, branched, or ring hydrocarbon structures, as well as isopentanol and farnesene, which are considered alternatives to gasoline (15). The skeletal complexity in terpenes, leading to the formation of such a diverse structure, is justified by an understanding of terpenoid biosynthesis.
Methodology
A comprehensive review of literature published between 2000 and 2025 was conducted to examine the biosynthesis, structural diversity, pharmacological significance, and industrial applications of terpenoids. A systematic search was performed using databases such as PubMed, Scopus, Web of Science, and Google Scholar. The search strategy employed specific combinations of keywords, including “terpenoid biosynthesis,” “MEP pathway,” “MVA pathway,” “terpenoid industrial applications,” “plant terpenoids,” “synthetic biology terpenes,” and “metabolic engineering of terpenoids.” The inclusion criteria encompassed peer-reviewed original research articles, review papers, and book chapters that provided insights into the biochemical pathways, molecular mechanisms, and applied aspects of terpenoid metabolism. Studies were excluded if they focused on unrelated classes of natural products, were not peer-reviewed, were not available in English, or lacked specific references to terpenoid pathways or applications. Emphasis was placed on recent publications (2010-2025) to reflect current advances in the field. Additionally, reference lists of key articles were manually screened to identify further relevant studies. Data extraction focused on elucidation of pathways, bioengineering strategies, compound diversity, and reported therapeutic or industrial uses, followed by a qualitative synthesis of significant trends, challenges, and gaps in current research.
Objectives
This review aims to examine recent advances in terpenoid biosynthesis, with a focus on both the MVA and MEP pathways. We discuss the enzymatic mechanisms that drive chemical diversity, identify emerging industrial applications and trends, and highlight both current challenges and future directions for research. By positioning this review within the broader context of growing global interest in sustainable bioresources and pharmaceutical innovation, we seek to provide insights into the untapped potential of terpenoids and to offer a roadmap for future investigations.
Terpenoid Biosynthesis in Plants
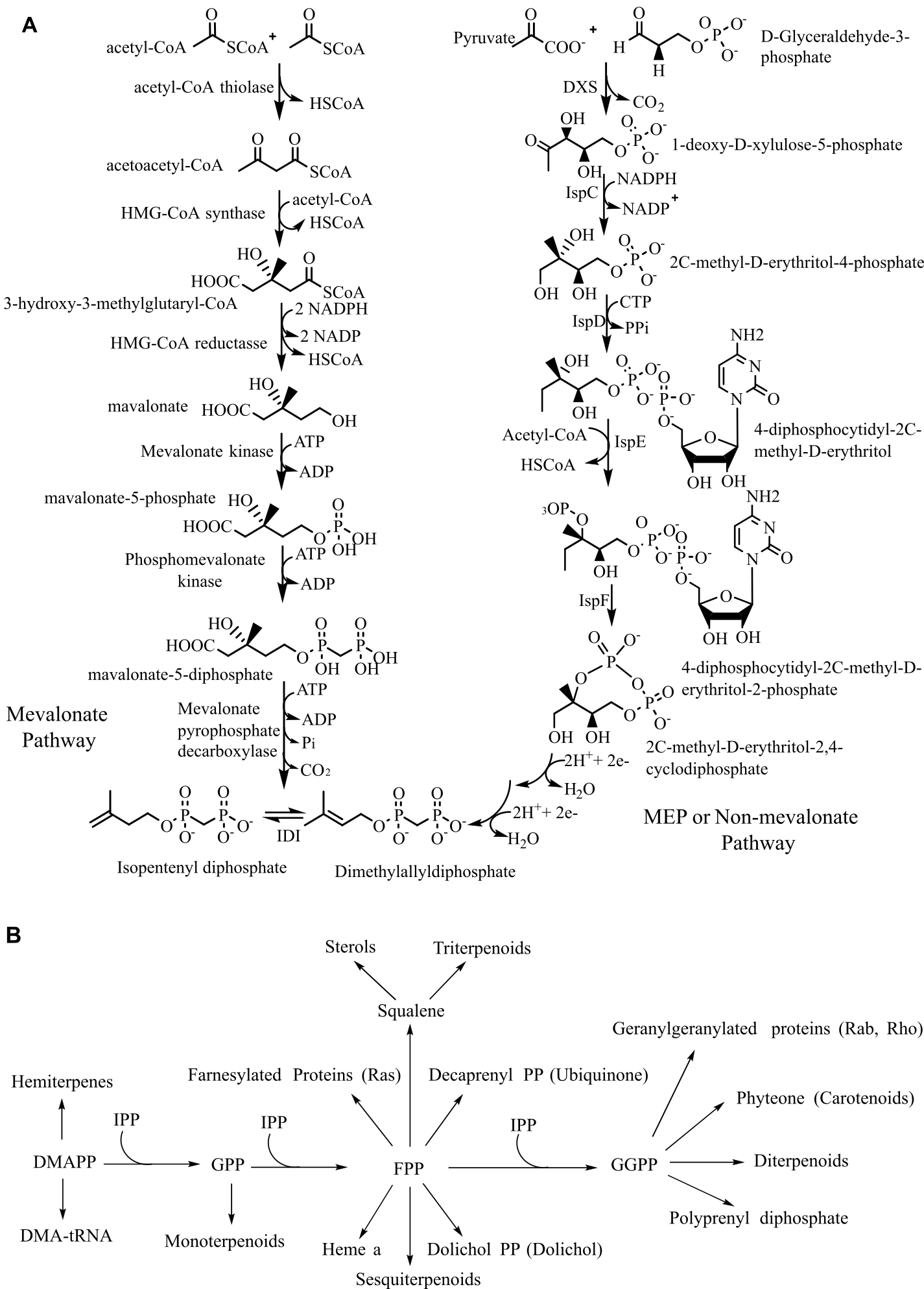
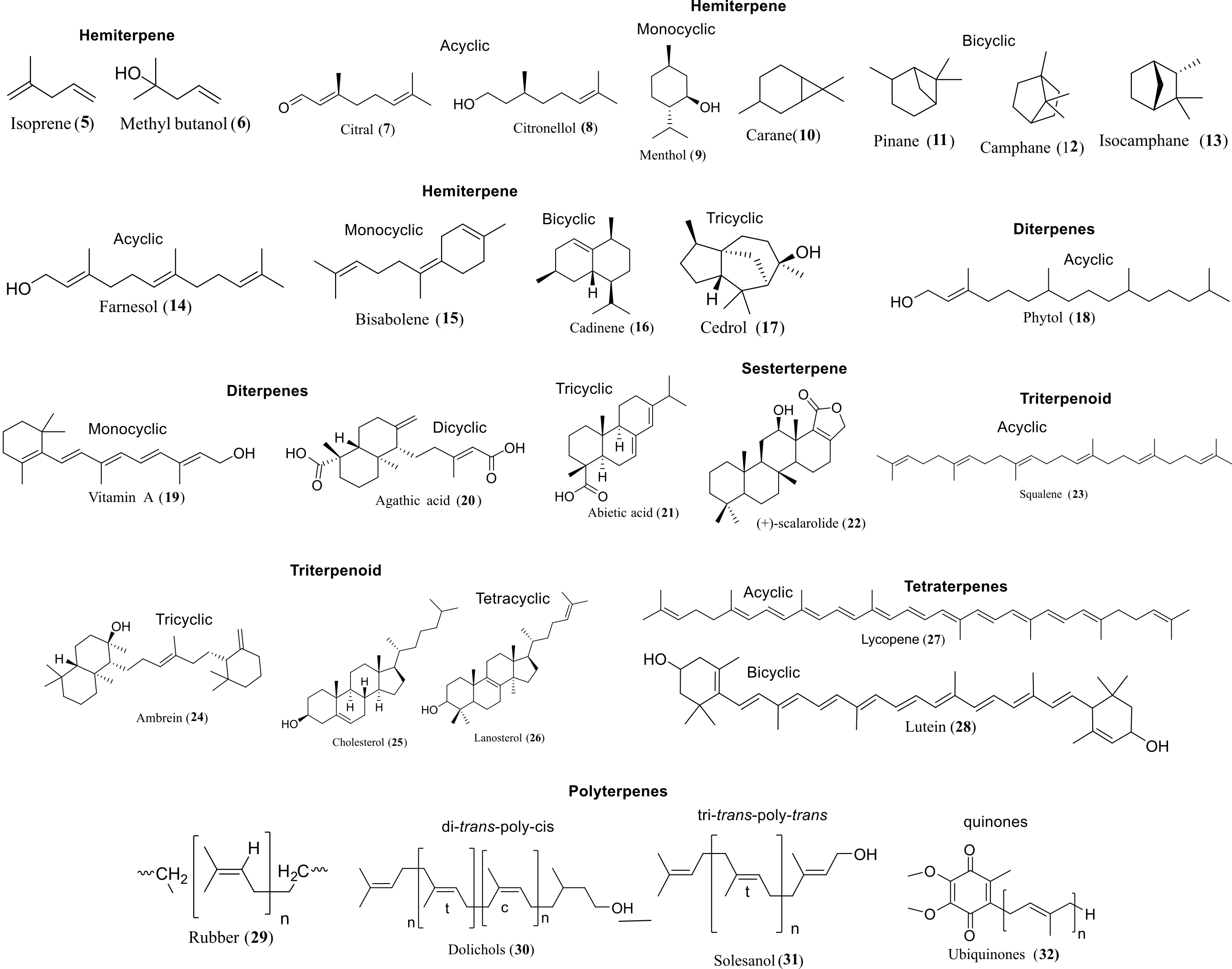
Biosynthesis of all isoprenoids begins with two simple five-carbon building blocks, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). They are classified by the number of five-carbon units present in the core structure (hemi, mono, sesqui, di, ses, tri, tetra, and poly-terpenes) (16). In all eukaryotes (mammals, fungi, plants), archaea, and some eubacteria, IPP and DMAPP are synthesized from three molecules of acetyl-CoA in seven steps by the mevalonate (MVA) or HMG-CoA reductase pathway, which operates in the cytosol and mitochondria (Figure 1A) (17).
Before the 1990s, it was believed that isoprenes are only synthesized by the MVA pathway. In the mid-1990s, Rohmer and Arigoni discovered the existence of a second pathway for IPP and DMAPP biosynthesis, which is now firmly established in eubacteria, cyanobacteria, apicoplast-type protozoa, and plants. This pathway starts with pyruvic acid, glyceraldehyde-3-phosphate (GAP), leading to the formation of IPP and DMAPP in seven enzymatic steps. In this biosynthesis, the first pathway-specific intermediate is 2-C-methyl-D-erythritol-4-phosphate (MEP), from which the pathway derives its name (18). In addition to isoprenoid synthesis, deoxy-xylulose phosphate (DXP) is the precursor for the biosynthesis of thiamin (vitamin B1) and pyridoxol (vitamin B6); hence, the pathway is also known as the 1-deoxy-D-xylulose 5-phosphate (DOXP) pathway (19).
Both the MVA and MEP pathways ultimately produce DMAPP, and from this point onward, a similar enzymatic machinery is used across organisms to generate the diverse array of terpenoid compounds found in nature (Figure 1B) (20). Hemiterpenes are the smallest known terpenes produced by most plants, and the first terpenes formed from DMAPP or IPP. The basic reaction in the isoprenoid pathway is chain elongation, where the allylic carbocation formed from isoprenoid allylic diphosphate, such as DMAPP, geranyl diphosphate (GPP), farnesyl diphosphate (FPP), is added to the double bond in IPP to form a growing allylic diphosphate chain product. The chain elongation or head-to-tail or 1'-4 linkage is by far the most common and occurs in all organisms (21). Terpenes are biosynthesized from isoprene units by four fundamental reactions: chain elongation, cyclopropanation, branching, and cyclobutanation. Chain elongation is the basic reaction in the terpene biosynthesis. Molecules generated by these reactions have head-to-tail or regular carbon skeletons. The other three reactions produce irregular carbon skeletons. Cyclopropanation (c1'-2-3) reaction is the first pathway-specific reaction in the biosynthesis of triterpenoids, steroids, which are formed through the cyclopropyl intermediate, pre-squalene (C30), and carotene compounds through pre-phytoene (C40) found in Archaea, some bacteria, and all Eukaryotes (22). Mealybug mating pheromones such as planococcyl and maconelliol are formed through irregular cyclobutation reaction (23).
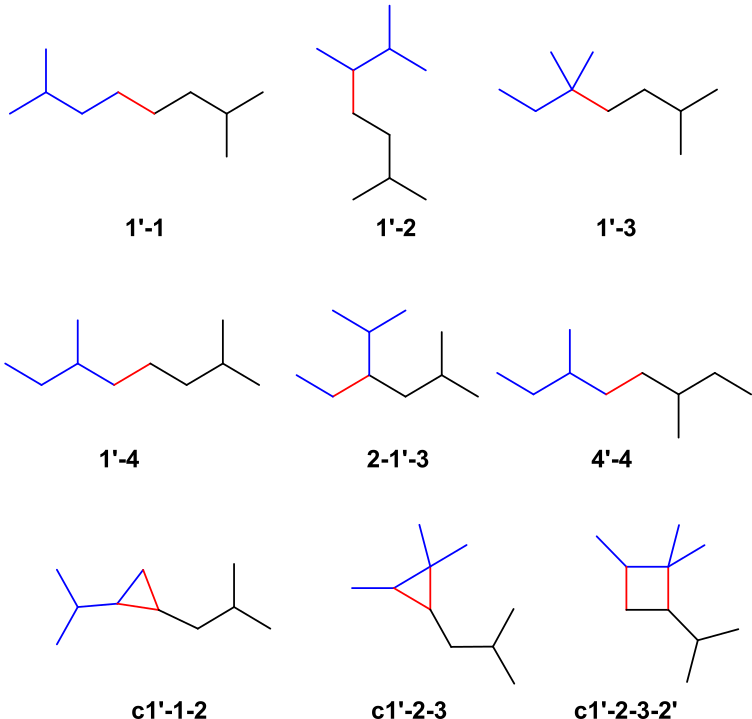
A 3-methyl-1-butyl is the basic unit of isoprenoid carbon skeletons (see Figure 2), which are joined in nine different patterns (1, 24). The four structures followed a combination in which C1’ of one unit (blue) formed a bond (red) with the single carbon of another unit (black) (1′-1, 1′-2, 1′-3, and 1′-4). C1′ is joined to the other unit in a cyclic structure (c1′-1-2, c1′-2-3, and c1′-2-3-2′). This pattern showed in three structures, and in one structure, C1′ is inserted between atoms in the other unit (2-1′-3). Only one structure, which follows the 4′-4 combining pattern, does not involve C1’. Regular terpenes commonly possess a 1′-4 linkage in the isoprene units. Other attachment patterns are designated as non-head-to-tail or irregular terpenes. Four of the basic isoprenoid structures (1′-2, 1′-4, c1′-2-3, and c1′-2-3-2′) are synthesized by joining simpler units, whereas the other four (1′-1, 1′-3, 2-1′-3, and c1′-1-2) are produced by rearrangement of 1′-2-3 structures. In the isoprenoid biosynthesis pathway, all the isoprenoid skeletons are formed before the modification reactions that are required for the synthesis of a specific natural product, except for the 4′-4 attachment pattern.
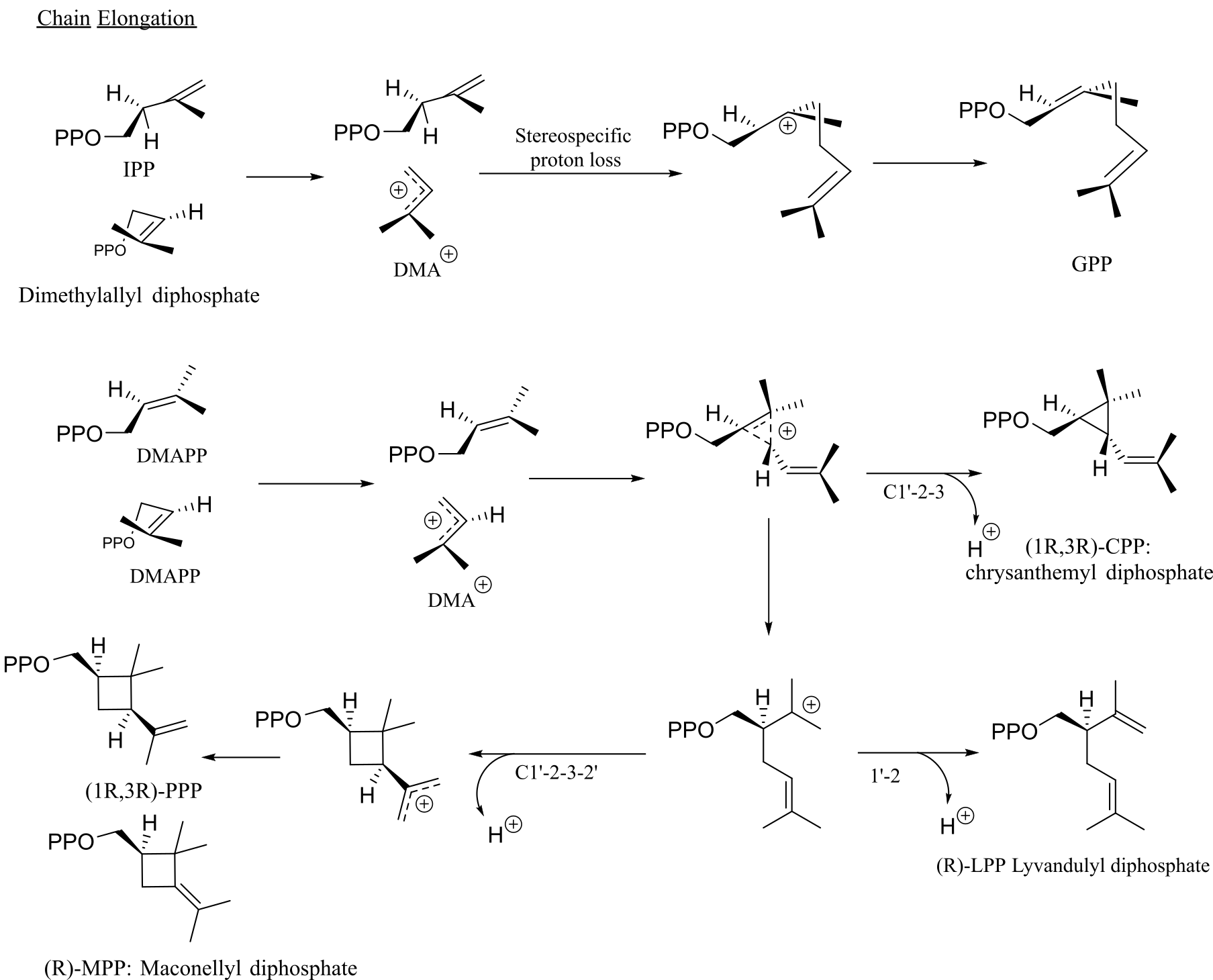
The mechanism for the four fundamental steps involved in terpenes biosynthesis is studied using deuterium isotope to trace the key rearrangement and elimination steps reported by Thulasiram et al. (25). In chain elongation, the first step of the reaction is the formation of the dimethylallyl cation (DMA+) from DMAPP. Further, the reaction proceeds by dissociative electrophilic alkylation of the IPP double bond by the cation (see Figure 3). Stereospecific deprotonation of the intermediate carbocation generates GPP. Chrysanthemyl cation (C+) is a protonated cyclopropane intermediate formed by the dissociative electrophilic alkylation of the double bond DMAPP by dimethyl allyl cation (DMA+) in C1’,4-addition. Further pro-S hydrogen lost from the C1 position of the intermediate leads to chrysanthemyl diphosphate (CPP). The rearrangement reaction in chrysanthemyl cation (C+) forms lanvandulyl cation (L+), in the branching step, which can be deprotonated to generate lavandulyl diphosphate (LPP). Cyclobutanation of the lanvandulyl cation (L+) generates a protonated cyclobutyl carbinyl cation intermediate. Deprotection of the intermediate cation leads to Maconellyl diphosphate (MPP).
Monoterpenoids are acyclic (linalool, myrcene, and ocimene), or monocyclic (limonene, carveol, and carvone), or bicyclic (camphene, sabinene, and pinene) compounds synthesized from GPP catalyzed by monoterpene synthases (see Figure 4). GPP binds to the active site of monoterpene synthases as a complex with the divalent metal ion. GPP undergoes ionization to form the linalyl cation, which then undergoes further isomerization and cyclization to create a diverse range of monoterpenes. In plants, monoterpenoids are synthesized through the MEP pathway.
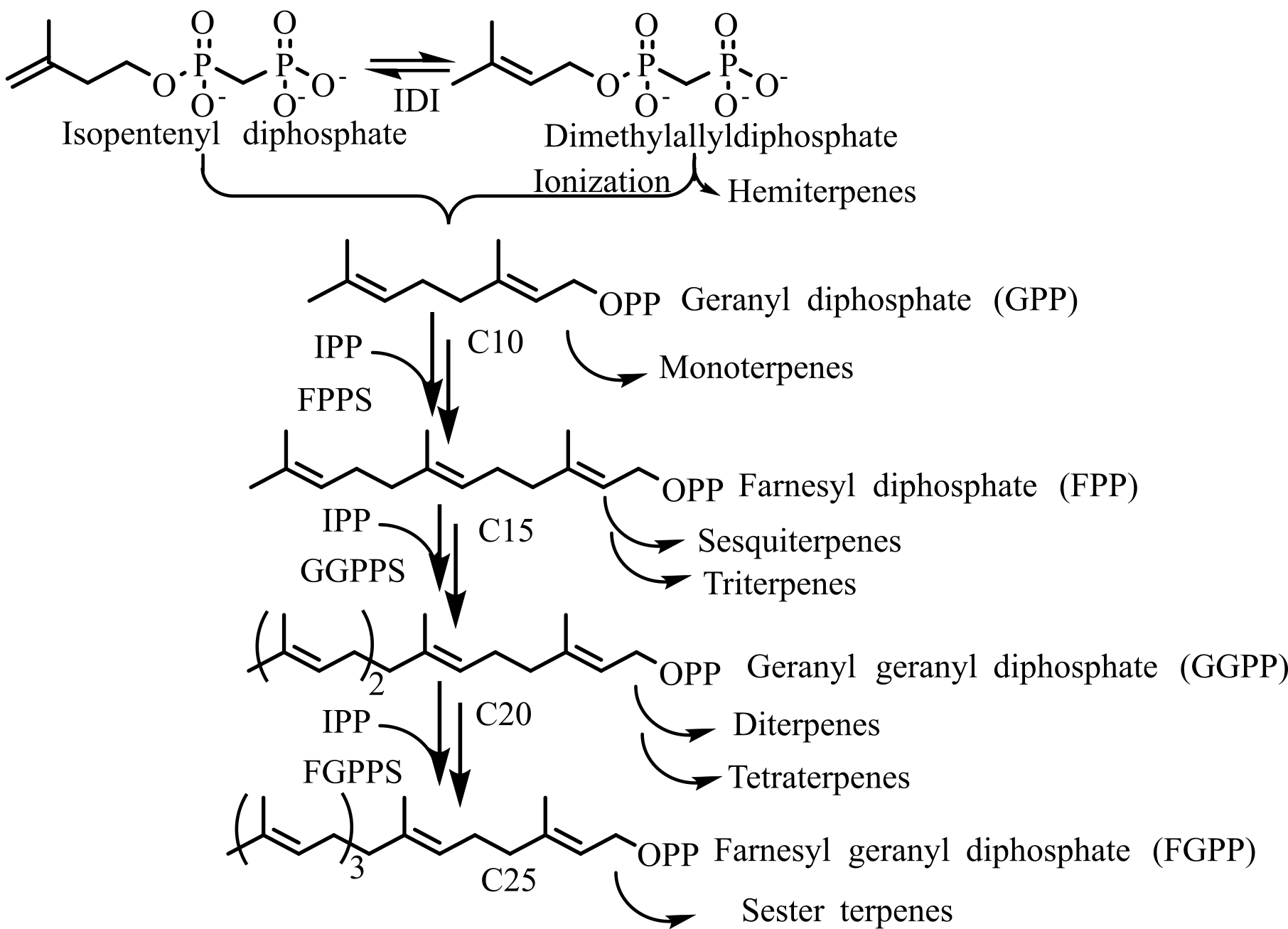
Farnesyl diphosphate (FPP), which lies at the juncture of the terpene pathway, branched into sesquiterpenes, squalene, farnesylated proteins (Ras), decaprenyl PP ubiquinones, heme a, and dolichol PP (dolichol). Sesquiterpenes are generated by the ionization of FPP, followed by intermolecular cyclization. Squalene synthase catalyzes 2 FPP condensation to form squalene, which is further oxidized to the (3S)-oxidosqualene/2,3-oxido squalene in the pathway, and it is the common substrate for the biosynthesis of all the triterpenoids. Triterpenoids are synthesized by cyclization of oxidosqualene, in which the first step is a 2,3-peroxide bond cationic attack, which generates a cyclic carbocation. 1,2-proton and methyl shifts of cation intermediates lead to the formation of different triterpenes. In plants, triterpenoids are stored in a glycosylated form, known as saponins. Modification of lanosterol and cycloartenol produces steroids and hormones. Lanosterol synthesis is present in animals and fungi for steroid synthesis, whereas cycloartenol synthase is involved in the steroid biosynthesis of plants, protozoa, and microalgae.
Diterpenoids are biosynthesized from GGPP, which is formed by cyclization followed by several skeletal modifications. Most diterpenoids are secondary metabolites and are typically limited to specific plant taxa as signature metabolites. Head-to-head condensation of two GGPP molecules forms phytoene, which is used as a precursor for tetraterpenoids. Polyprenoids are the linear polymers produced from isoprene (6-100) with a primary alcohol at a terminal. The reduction of a double bond at the α-residue results in the formation of dolichols. Dolichols are polyprenoids produced by bacteria and plants, and are involved in N-glycosylation of proteins. Quinones are one of the critical groups in polyprenoids, containing a polar head group and a non-polar isoprenoid side chain. These quinones are involved in the electron transport chain. Rhodoquinone (RQ), the ubiquinone derivative with an amino group substituting for a methoxy group, is present in purple bacteria of the Rhodospirillaceae family.
Advances In Synthesis
Terpenoids have potential benefits in various sectors, hence the demand is rapidly increasing (26-28). Low yields and high costs restrict the direct extraction from plants and other naturally occurring sources. Synthetic production, an alternative source for terpenoid synthesis, produces several isomers (29-31).
Metabolic engineering offers a powerful approach to optimize host organisms for the efficient biosynthesis of specialized terpenoids by rewiring native metabolic pathways and introducing non-native modules. Central to this strategy is the redirection of carbon flux toward the mevalonate (MVA) and 2-C-methyl-D-erythritol 4-phosphate (MEP) pathways, which generate the universal terpenoid precursor isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Enhancing precursor supply has been a primary target in model organisms like Escherichia coli and Saccharomyces cerevisiae. For example, overexpression of MVA pathway enzymes and optimization of regulatory networks in S. cerevisiae significantly boosted monoterpenoid and sesquiterpenoid production (32, 33). Similarly, engineered E. coli strains expressing heterologous MVA or MEP pathways demonstrated enhanced production of compounds like amorpha-4,11-diene and artemisinic acid (34).
Additionally, balancing the expression of rate-limiting enzymes, minimizing the accumulation of toxic intermediates, and compartmentalizing biosynthetic steps have proven effective. For instance, the introduction of dynamic control systems and feedback-insensitive enzymes improved titers and pathway stability (35). Co-expression of cytochrome P450 monooxygenases and their redox partners is also essential for producing oxygenated terpenoids, such as tanshinones and taxol intermediates (36). Host engineering strategies, including the deletion of competitive pathways and the engineering of organelles (e.g., peroxisomes or mitochondria), further enhanced product yield and purity. Collectively, these metabolic engineering strategies enable the precise modulation of complex biosynthetic networks, allowing for the production of high-value, specialized terpenoids at industrial scales (37)
Protein engineering has emerged as a pivotal strategy for enhancing microbial cell factories in the biosynthesis of terpenoids, particularly by optimizing the function of key enzymes such as terpene synthases and prenyltransferases. These enzymes, in their native forms, often lack the required specificity, stability, and catalytic efficiency for industrial-scale production. To address these limitations, various protein engineering techniques, including directed evolution, structure-guided engineering, and rational design, have been applied. For instance, directed evolution coupled with site-saturation mutagenesis led to the development of a triple-mutant isopentenyl diphosphate isomerase (IDI) variant (L141H/Y195F/W256C) with a 2.53-fold increase in catalytic activity, which resulted in 2.8-fold more lycopene production in engineered strains (38, 39). Similarly, error-prone PCR was used to create improved isoprene synthase (ISPS) mutants, achieving a threefold increase in isoprene yields.
Another essential consideration is enzyme promiscuity, a common feature of terpenoid synthases that often leads to the production of undesirable by-products. For example, levopimaradiene synthase (LPS) was engineered through combinatorial mutation approaches, resulting in a variant that produced a ~2600-fold increase in levopimaradiene while minimizing isomeric side products (40). In the case of Taxol biosynthesis, less than 10% of the precursor taxadiene is naturally converted into the desired hydroxylated product. Site-saturation mutagenesis of taxadiene synthase successfully shifted the pathway toward preferred intermediates, enhancing taxadiene-4(20)-11(12)-diene production by 2.4-fold (41).
Despite these advances, several challenges persist in protein engineering for terpenoid biosynthesis. The lack of comprehensive structural and functional annotation of enzymes restricts rational design efforts. Furthermore, fused proteins can suffer from issues such as misfolding, reduced catalytic efficiency, and low expression. These challenges underscore the need for more detailed studies on domain-linker interactions to facilitate the design of fusion proteins with greater precision and effectiveness. Overall, protein engineering remains a powerful and indispensable tool in the development of efficient microbial cell factories for terpenoid production (37).
Gene clustering plays a vital role in the regulation and biosynthesis of specialized terpenoids by co-localizing biosynthetic genes into operon-like structures in microbial and plant genomes. These clusters enhance metabolic efficiency by enabling co-regulation, coordinated expression, and reduced metabolic crosstalk. Although gene clustering is well-established in microbial systems, emerging evidence also highlights its presence in plant genomes. For instance, gene clusters responsible for the biosynthesis of compounds such as momilactone, phytocassane, and oryzalexin have been identified in Oryza sativa (rice), where key enzymes, including terpene synthases and cytochrome P450s, are spatially organized to facilitate efficient biosynthetic flux (42, 43). Similar clusters are involved in the biosynthesis of thalianol and marneral in Arabidopsis thaliana (44). The existence of these gene clusters suggests an evolutionary advantage, as co-localization may arise through gene duplication and divergence followed by neofunctionalization within specific genomic regions. Furthermore, synthetic biology has leveraged this natural architecture to reconstruct and express entire gene clusters in heterologous hosts, such as yeast or E. coli, thereby improving yields and pathway control. These efforts demonstrate the utility of gene clustering not only as an evolutionary strategy but also as a practical tool for designing efficient cell factories for terpenoid production.
Classification of Terpenoids
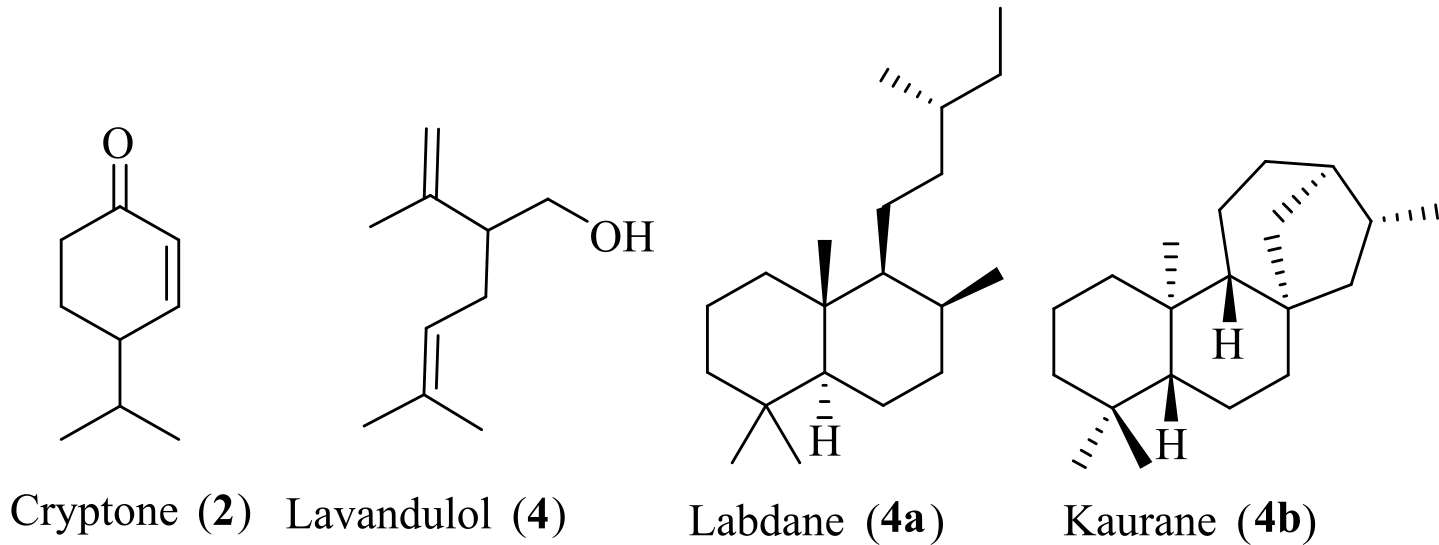
Terpenoids are classified according to the number of isoprene units present in them, e.g., hemiterpenes, monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, polyterpenes (see Table 1) (45). However, in some cases, there are violations of the isoprene rule, such as cryptone (2) and lavandulol (3) (46). The carbons contained in cryptone, a naturally occurring ketonic terpenoid, are not a multiple of five, and it contains only nine carbons. Lavandulol is composed of two isoprene units, linked through an exceptional C3 and C4 (3,4-linkage). Hemiterpenes are the smallest and simplest terpenes, consisting of a five-carbon single isoprene unit; they have low occurrence in nature with no significant bioactivities, e.g., methyl butanol (5) (47). Monoterpenes are reclassified based on rings present in their structure as acyclic (citral (7), citronellol (8)), monocyclic, e.g, menthol (9), α-terpineol, and bicyclic. Bicyclic monoterpenes are further reclassified into three classes according to the second ring size, such as 6+3 (carane) (10), 6+4 (pinane) (11), 6+5 (camphane) (12) (48). Sesquiterpenoids are classified according to the number of rings present in their acyclic (farnesol (14)), monocyclic (bisabolene (15)), bicyclic (cadinene (16)), and tricyclic (cedrol (17)) (49). The diterpenes are a diversified class of terpenoids, biosynthesized by the combination of four isoprenoid units. Diterpenoids are classified according to the number of rings present in the structure: acyclic (phytol (18)), monocyclic (vitamin A (19)), bicyclic (agathic acid (20)), tricyclic (abietic acid (21)) (50).
Diterpenoids are further subdivided according to the specific skeletal structures, like labdanes (4a), ent-kaurans (4b), halimanes, clerodanes, pimaranes, and abeitanes (see Figure 5). Labdane is a bicyclic diterpenoid; the name originated from the labdanum, a resin derived from the gum rockrose. ent-kauran is a bioactive class of tetracyclic diterpenoids. Five isoprene-containing sesterterpenes are rare compounds that are mainly isolated from marine sponges. Triterpenes are biosynthesised from six isoprene units and are a biologically important class of terpenes. It is classified into acyclic (squalene (23)), tricyclic (ambrein (24)), and tricyclic (lanosterol (26)). Tetraterpenes contain eight isoprene units and are classified into acyclic (lycopene (27)) and bicyclic (lutein (28)).
Terpenes | Isoprene units | Number of carbons |
Hemiterpenes | 1 | 5 |
Monoterpenes | 2 | 10 |
Sesquiterpenes | 3 | 15 |
Diterpenes | 4 | 20 |
Sesterterpenes | 5 | 25 |
Triterpenes | 6 | 30 |
Tetraterpenes | 8 | 40 |
Polyterpenes | >8 | >40 |
Carotenoid pigments are a crucial cyclic class of tetraterpenes used as a natural food additive and in the pharmaceutical industry. Xanthophyll pigments are the other broadly distributed class of tetraterpenes, responsible for flowers, vegetables, and fruit colouring (52). Polyterpenes are the polymer of isoprene units (6-100) with the hydroxyl group at the terminal. Rubber (29) is an extensively used polyterpene for various human applications. It is present in several plants in the latex form, e.g., Hevea brasiliensis (Rubber tree). It is classified into di-trans-poly-cis (dolichols) and tri-trans-poly-trans (solanesol (31)). Quinones are one of the critical groups in polyprenoids, containing a polar head group and a non-polar isoprenoid side chain.
Terpenoids Applications
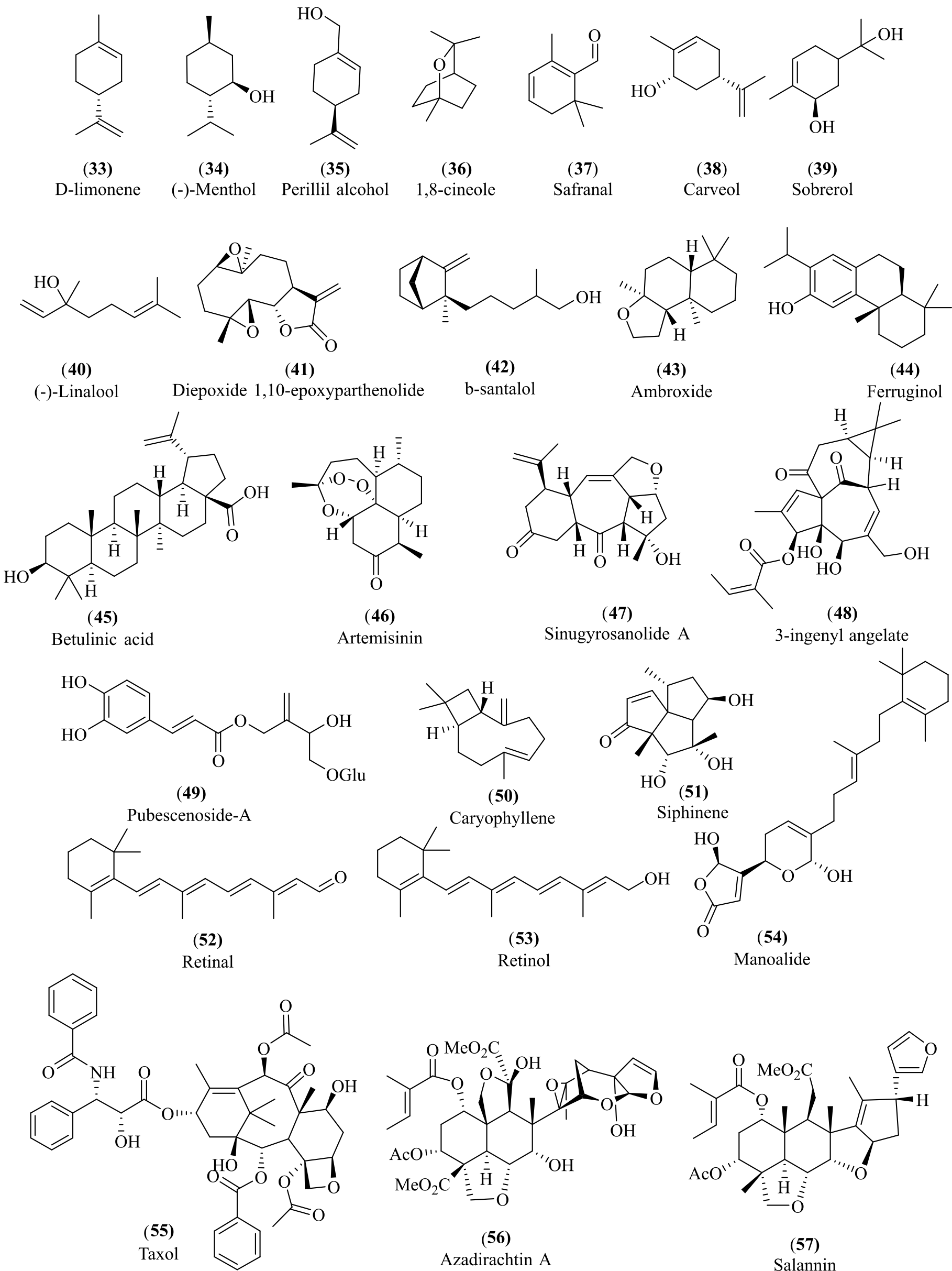
Terpenoids represent a diverse class of molecules with complex structures and functionalities, providing significant opportunities to address various human social and health issues. Terpenoids exhibit vast structural diversity and multifunctionality, which underpin their broad spectrum of industrial applications (53). Based on an extensive survey of literature and recent biotechnological advancements, terpenoids have been found to play pivotal roles across multiple sectors, including pharmaceuticals, agriculture, energy, food, cosmetics, and synthetic biology. Numerous terpenoids are pharmaceutically active, displaying a wide range of bioactivities, including anticancer, antimalarial, antibacterial, and anti-inflammatory effects. (54, 55). Terpenes, viz. ambroxides and santalol, play an essential role in the fragrance industry in perfumery essential oils (see Figure 6) (56). Terpenoids have pharmacological properties, including anticancer, antimicrobial, antiallergenic, antihyperglycemic, antispasmodic, and anti-inflammatory properties (57, 58).
Hemiterpenes are the smallest terpenes with no significant bioactivities. Pubescenoside-A, a hemiterpene glycoside isolated from Ilex pubescens, is reported to exhibit anti-platelet aggregation activity (59). Monoterpenes produced in plants act as a chemical defense against herbivores, as fragrances to attract pollinators, and also asphytotoxins to other plants (60). Limonene at a concentration of 10 µM acts on the microtubules, leading to membrane leakage in the transgenic Arabidopsis thaliana weed (61, 62). Limonoids from Meliaceae family trees are used for pest management due to their antifeedant and insecticidal properties (63). In the food industry, terpenoids are used as color, flavor, and fragrance enhancers. 1-Menthol (34) is one of the most important flavoring agents in pharmaceuticals, cosmetics, toothpaste, chewing gum, and other personal care products, as well as in cigarettes (64). 1,8-cineole (36) is commonly used in the flavouring and fragrance industries due to its pleasant taste and spicy aroma (65-67). Cineole-based eucalyptus oil is used as a flavouring agent at low levels (0.002 %) in various products, including baked goods, confectionery, meat products, and beverages (67). Safranal (37), biosynthesized from the carotenoid degradation pathway, is one of the most expensive spice flavour compounds present in Saffron (Crocus sativus L.) (68). D-limonene (33) and perillyl alcohol (35) are known to inhibit the development of carcinomas in the liver, mammary, skin, lung, colon, prostate, and pancreas (69, 70). Monoterpenes such as carveol (38) and sobrerol (39) have shown activity against mammary carcinomas (71). Menthol exhibited a bacterial activity against S. aureus and E. coli. (72). Sesquiterpenes are less volatile compared to hemiterpenes and monoterpenes and function as semichemicals in plants and insects (73). It has several pharmacological activities such as anti-inflammatory, bactericidal, antioxidant, and antitumor activities (74). β-Caryophyllene sesquiterpenes are the first known “dietary cannabinoid” present in spices and have generally been recognized as safe by the FDA as a standard food component. β-caryophyllene in cancerous cell lines stimulates apoptosis and suppresses growth (75). Silphinenes and their derivatives exhibited insect antifeedant activity against aphid, and Leptinotarsa decemlineata species (76)
Sinugyrosanolide-A is a trans-neoclerodane type diterpene and a psychoactive drug, ferruginol (44), has been reported to possess anti-mycobacterial activity (10, 77). 3-ingenyl angelate (48), a hydrophobic diterpene ester isolated from Euphorbia peplus, is reported to be used as a topical chemotherapeutic agent for skin cancer (78). Sesterterpenes are rare in nature compared to other terpenes, and a maximum of them was isolated from marine fungi. Manoalide (54), a bioactive sesterterpene isolated from Luffarella variabilis sponge. It exhibited various bioactivities, including antihypertensive (calcium channel blocker), anti-inflammatory, antimicrobial, and analgesic properties (79). Betulinic acid (45) has been shown to induce apoptosis of several human tumor cells, including melanoma and glioma. It is used as an inhibitor for the NF-κB pathway (80, 81).
As discussed above, terpenoids are bioactive molecules with structural and chemical diversity, providing opportunities to discover new drug leads (82, 83). An in silico docking study reveals the structural features in triterpenoids that are crucial for antimycobacterial activity, highlighting their potential as scaffolds in anti-tuberculosis drug discovery (84). The isolation and purification of crude extracts facilitate the characterization of bioactive constituents, which may aid in the synthesis of large quantities, derivative formation, determination of the mechanism of action, formulation as a drug, and derivatization. Additionally, bioassays can be developed for pure bioactive molecules, which is crucial for screening them for various bioactivities and ensuring quality control in therapeutic formulations for consumption as a drug (85). Comprehensive review of pharmacological activities of bioactive compounds, including terpenoids, isolated from Acacia pennata, with in silico repurposing perspectives (53). In nature, the plants are the primary sources of terpenoids. In folk or traditional medicine, medicinal plant parts, powders, or crude extracts were used for therapeutic and experimental purposes. However, the use of these crude preparations has several disadvantages, including variations in the quantity of bioactive constituents, challenges in raw material collection, storage, and preparation (86, 87). Ethanolic extracts of Mentha viridis rich in terpenoids show significant antidiabetic and antihyperlipidemic effects, especially when combined with metformin (88). Comparative phytochemical analysis confirms the abundance of terpenoids in Azadirachta indica and Vernonia amygdalina, supporting their broad medicinal applications (89).
Review highlights neuromodulatory effects of plant secondary metabolites, including terpenoids, in prevention and management of neurological disorders (90). Triterpene glycoside glycyrrhizin and glycyrrhetinic acid act via the PI3K/Akt/GSK3-β pathway to reduce cytoline production, hence investigating their therapeutic potential for asthma treatment (91). Natural rubber is the isoprene polymer, which is extensively used for various applications, either alone or in combination with other materials (92). Pandanus amaryllifolius leaf extracts, containing triterpenoids/steroids, exhibit notable antibacterial and antifungal activities, making them suitable for anti-dandruff shampoo formulations (93). The isolation of triterpenoid and steroidal cinnamates from Vitellaria paradoxa demonstrates a strong antibacterial potential against resistant pathogens (94). Fractions of Detarium microcarpum lacking terpenoids but rich in other phytochemicals display promising antioxidant activities (95). MPLC is a robust, automated technique for developing an effective, rapid, and reproducible protocol for the preparative-scale isolation of natural products. Ultra-performance liquid chromatography coupled with high-resolution mass spectrometry is a technique that enables the recognition of expected metabolites in complex plants or biological mixtures, even at minor concentrations (nm-pm range), with low sample requirements, high sensitivity, rapidity, and high accuracy (96, 97). Aquilaria malaccensis leaves containing triterpenoids accelerate wound healing in second-degree burns, with higher concentration ointments proving more effective (98). Ethanolic extract of Piper ornatum leaves, containing terpenoids, shows potent larvicidal activity against mosquito larvae (99). A comprehensive review on the pharmacological activities and industrial potential of terpenoids and other bioactive compounds isolated from Acacia pennata, including their chemical diversity and emerging therapeutic applications (53).
Plant extraction protocols play the most crucial role in the isolation and characterization of bioactive terpenoids. The earlier extraction protocol was solely focused on the analytical-scale isolation of a few targeted compounds; therefore, an alternative technique is required that facilitates the easy availability of these compounds on a large scale (100, 101). Explores the antiepileptic potential of Amaranthus spinosus methanol extract, highlighting the presence of terpenoids among other phytochemicals, suggesting therapeutic relevance, but not focused on biosynthesis or industrial applications (102). Assesses quality control of a polyherbal medicine (Paxherbal Bitter Tea) using thin-layer chromatography and phytochemical profiling, identifying terpenoids among key components without addressing biosynthetic advances (103).
However, terpenoid bioactive compounds have not been explored efficiently due to their low bioavailability, which significantly affects their bioactivities (104, 105). The application of surfactant to enhance the solubility of hydrophobic bioactive terpenoids in water, due to their amphiphilic behavior, which tends to reduce the surface tension of drugs, is one of the strategies to improve bioavailability (106). Biotransformation is a green approach used to expand the chemical diversity of terpenoids under mild reaction conditions (107-109). Commonly used terpenoids can be seen in Figure 7.
Industrial Important Terpenoids
Clinically approved drugs, such as paclitaxel (a diterpenoid) and artemisinin (a sesquiterpenoid), are prominent examples, widely used in cancer chemotherapy and malaria treatment, respectively (110, 111). In addition, menthol, thymol, and camphor are employed in analgesics and antiseptics, reinforcing the role of terpenoids in over-the-counter therapeutic formulations (112-114). The discovery of these bioactivities has led to increased interest in terpenoid biosynthesis for the development of new drugs. The aroma and flavor industries extensively utilize monoterpenes and sesquiterpenes, which contribute to the characteristic scents and flavors of many plant-derived products (115, 116). Compounds such as limonene, linalool, and farnesol are commonly used in perfumes, essential oils, and food flavorings (117). The vital oils of species like Eucalyptus globulus, Mentha spp., and Cymbopogon citratus owe their commercial value largely to their terpenoid content. This widespread use underscores the economic significance of volatile terpenoids. Terpenoids function as natural agrochemicals due to their role in plant defense mechanisms (118). Compounds such as pyrethrins (derived from Chrysanthemum) and azadirachtin (extracted from Azadirachta indica) exhibit strong insecticidal properties and are incorporated into eco-friendly biopesticides. Their biodegradability and lower toxicity to non-target organisms make them suitable alternatives to synthetic pesticides (119). Furthermore, terpenoid-based semiochemicals are being explored for pest deterrence and crop protection strategies. Recent advances in synthetic biology have enabled the microbial production of terpenoids with properties similar to those of fuels (120). Compounds such as isoprene, pinene, and farnesene are being investigated as renewable alternatives to petroleum-derived fuels due to their high energy density and combustion characteristics (121). In the food industry, terpenoids are utilized as flavor enhancers, colorants, and nutritional supplements. β-Carotene, a tetraterpenoid, is widely used as a natural food colorant and as a precursor to vitamin A. Other terpenoids, such as carvone and limonene, impart characteristic flavors to beverages, confectionery, and seasonings. The safety and stability of these compounds have further accelerated their adoption in food formulations (55, 122). Several terpenoids are incorporated into cosmetic formulations due to their aromatic, antimicrobial, and skin-soothing properties (112, 123). For instance, squalene, a triterpenoid, serves as an emollient and antioxidant in skincare products. Menthol and camphor are valued for their cooling and anti-irritant effects (124). The increasing demand for natural and plant-based cosmetics has driven market growth in terpenoid-derived personal care products. Beyond their bioactivity, terpenoids serve as chemical intermediates and biopolymers. Natural rubber, composed of polyisoprene, is a polyterpenoid with extensive use in the automotive and manufacturing industries (125). Additionally, terpenoid alcohols and aldehydes, such as geraniol and citral, are employed as starting materials in fine chemical synthesis (126). Advancements in metabolic engineering and synthetic biology have enabled scalable and sustainable production of high-value terpenoids (127). Terpenoids are being explored for roles in nanoparticle synthesis, drug delivery systems, and bio-based plastics. Their tunable structures and biocompatibility open new avenues in green chemistry and biomedical engineering (128, 129).
Conclusion
Terpenoids represent one of the most structurally and functionally diverse classes of natural products, with immense biological and industrial significance. Their biosynthesis through the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways highlights the intricate enzymatic processes that contribute to their chemical diversity. From primary metabolites essential for cellular functions to secondary metabolites involved in plant defense and ecological interactions, terpenoids play indispensable roles in nature.
The broad range of industrial applications, spanning pharmaceuticals, fragrances, and biofuels, underscores their potential in sustainable biotechnology. The successful commercialization of terpenoid-based drugs, such as Taxol and artemisinin, demonstrates their relevance in modern medicine. At the same time, advancements in metabolic engineering and synthetic biology continue to expand their utility. Moreover, the increasing interest in terpenoids as renewable biofuel alternatives further highlights their potential in addressing global energy challenges.
Future research in terpenoid biosynthesis, particularly through biotechnological innovations, holds promise for enhancing production yields, diversifying structural modifications, and optimizing their industrial applications. By integrating insights from molecular biology, bioengineering, and industrial biotechnology, continued advancements in this field will pave the way for novel applications and sustainable production strategies.
This review highlights the importance of further investigating terpenoid biosynthesis and its industrial applications, ultimately contributing to scientific advancements and economic growth across various sectors.
Declarations
Acknowledgment
We express our sincere gratitude to Dr. Bhagwan Dongre, Principal of Moreshwar Arts, Science, and Commerce College, Bhokardan, for his invaluable encouragement and support.
Ethics Statement
Not relevant
Data Availability
Not applicable.
Funding Information
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflicting interest.
References
- Christianson DW. Structural and Chemical Biology of Terpenoid Cyclases. Chem Rev. 2017;117:11570–11648. doi: 10.1021/acs.chemrev.7b00287.
- Blank PN, Shinsky SA, Christianson DW. Structure of Sesquisabinene Synthase 1, a Terpenoid Cyclase That Generates a Strained [3.1.0] Bridged-Bicyclic Product. ACS Chem Biol. 2019;4:1011–1019.
- Yuan Y, Lei Y, Xu M, Zhao B, Xu S. Bioactive Terpenes from Marine Sponges and Their Associated Organisms. Mar Drugs. 2025.
- Tegegne BA, Begashaw T, Belay WY, Tariku MK, Zeleke TK, Jemal M, Getinet M, Alehegn AA, Dagne A. Kleinia (Asteraceae): comprehensive review of ethnomedicinal uses, phytochemical profiles, ethnopharmacological applications, and toxicological insights. Front Pharmacol [Internet]. 2025;15.
- McGarvey DJ, Croteau R. Terpenoid Metabolism. Plant Cell. 2007;7:1015. doi: 10.2307/3870054.
- Khatoon M, Dubey A, Janhvi K. Unveiling Anthraquinones: Diverse Health Benefits of an Essential Secondary Metabolite. Recent Pat Biotechnol [Internet]. 2024 [cited 2025 Mar 3];19:179–197. doi: 10.2174/0118722083301761240628083511.
- Mao Y-B, Chen X-Y, Wang L-J, Cheng A-X, Lu S, Lou Y-G. Plant Terpenoids: Biosynthesis and Ecological Functions. J Integr Plant Biol. 2007;49:179–186. doi: 10.1111/j.1744-7909.2007.00395.x.
- Harman JC. CHAPTER 21 - Herbal Medicine in Equine Practice. In: Wynn SG, Fougère BJ, editors. Veterinary Herbal Medicine. Saint Louis: Mosby; 2007. p. 411–439.
- Vranová E, Coman D, Gruissem W. Structure and Dynamics of the Isoprenoid Pathway Network. Mol Plant. 2012;5:318–333. doi: https://doi.org/10.1093/mp/sss015.
- Roman Paduch1, Martyna Kandefer−Szerszeń1 MT and JF. Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp. 2007;55:315. doi: 10.1007/s00005-007-0039-1.
- Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: natural inhibitors of NF-$κ$B signaling with anti-inflammatory and anticancer potential. Cellular and Molecular Life Sciences. 2008;65:2979–2999. doi: 10.1007/s00018-008-8103-5.
- Enan E. Insecticidal activity of essential oils: octopaminergic sites of action. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2001;130:325–337. doi: https://doi.org/10.1016/S1532-0456(01)00255-1.
- Monsarrat B, Mariel E, Cros S, Garès M, Guénard D, Guéritte-Voegelein F, Wright M. Taxol metabolism. Isolation and identification of three major metabolites of taxol in rat bile. Drug Metabolism and Disposition. 1990;18:895–901.
- Lévesque F, Seeberger PH. Continuous-Flow Synthesis of the Anti-Malaria Drug Artemisinin. Angewandte Chemie International Edition. 2012;51:1706–1709. doi: 10.1002/anie.201107446.
- Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–328.
- Bohlmann J, Keeling CI. Terpenoid biomaterials. Plant Journal. 2008;3:656–669. doi: 10.1111/j.1365-313X.2008.03449.x.
- Kuzuyama T. Mevalonate and Nonmevalonate Pathways for the Biosynthesis of Isoprene Units. Biosci Biotechnol Biochem. 2003;66:1619–1627. doi: 10.1271/bbb.66.1619.
- Zhao L, Chang W, Xiao Y, Liu H, Liu P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu Rev Biochem. 2013;82:497–530. doi: 10.1146/annurev-biochem-052010-100934.
- Sprenger GA, Schörken U, Wiegert T, Grolle S, de Graaf AA, Taylor S V, Begley TP, Bringer-Meyer S, Sahm H. Identification of a thiamin-dependent synthase in <em>Escherichia coli</em> required for the formation of the 1-deoxy-<span class="sc">d</span>-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proceedings of the National Academy of Sciences. 1997;94:12857 LP – 12862. doi: 10.1073/pnas.94.24.12857.
- Kuzuyama T, Seto H. Two distinct pathways for essential metabolic precursors for isoprenoid biosynthesis. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:41–52. doi: 10.2183/pjab.88.41.
- Thulasiram H V., Erickson HK, Poulter CD. A common mechanism for branching, cyclopropanation, and cyclobutanation reactions in the isoprenoid biosynthetic pathway. J Am Chem Soc. 2008;130:1966–1971. doi: 10.1021/ja0771282.
- Raja R, Hemaiswarya S, Rengasamy R. Exploitation of Dunaliella for $β$-carotene production. Appl Microbiol Biotechnol. 2007;74:517–523. doi: 10.1007/s00253-006-0777-8.
- Zou Y, Millar JG. Chemistry of the pheromones of mealybug and scale insects. Nat Prod Rep. 2015;32:1067–1113. doi: 10.1039/C4NP00143E.
- Dale Poulter C. Biosynthesis of Non-Head-to-Tail Terpenes. Formation of l′-l and 1′-3 Linkages. Acc Chem Res. 1990;23:70–77. doi: 10.1021/ar00171a003.
- Thulasiram H V., Erickson HK, Poulter CD. Chimeras of Two Isoprenoid Synthases. Science (1979). 2007;73:73–77. doi: 10.1126/science.1137786.
- Masyita A, Mustika Sari R, Dwi Astuti A, Yasir B, Rahma Rumata N, Emran T Bin, Nainu F, Simal-Gandara J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X. 2022;13. doi: 10.1016/j.fochx.2022.100217.
- Shataer D, Chang Y, Obul M, Aierken K, Liu H. An Up-to-date Review on the Classification, Pharmacology, and Production of Terpenes and Terpenoids. Curr Org Chem. 2025;29:1508–1522. doi: 10.2174/0113852728373242250319054222.
- Khanam S, Mishra P, Faruqui T, Alam P, Albalawi T, Siddiqui F, Rafi Z, Khan S. Plant-based secondary metabolites as natural remedies: a comprehensive review on terpenes and their therapeutic applications. Front Pharmacol. 2025;16. doi: 10.3389/fphar.2025.1587215.
- Roque R, Rodriguez V, Santos PM. Microbial Production of Menthol. Microbial Production of Food Bioactive Compounds. Cham: Springer Nature Switzerland; 2025. p. 1–26.
- Wang M, Zhang Z, Liu X, Liu Z, Liu R. Biosynthesis of Edible Terpenoids: Hosts and Applications. Foods. 2025;14:673. doi: 10.3390/foods14040673.
- González-Hernández RA, Valdez-Cruz NA, Trujillo-Roldán MA. Factors that influence the extraction methods of terpenes from natural sources. Chemical Papers. 2024;78:2783–2810. doi: 10.1007/s11696-024-03339-z.
- Ward VCA, Chatzivasileiou AO, Stephanopoulos G. Metabolic engineering of Escherichia coli for the production of isoprenoids. FEMS Microbiol Lett. 2018;365. doi: 10.1093/femsle/fny079.
- Sethia P, Ahuja M, Rangaswamy V. Metabolic Engineering of Microorganisms to Produce Isoprene. J Microb Biochem Technol [Internet]. 2019;11:419. doi: 10.4172/1948-5948.1000419.
- Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051.
- Zhou J, Wang C, Yang L, Choi E-S, Kim S-W. Geranyl diphosphate synthase: An important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzyme Microb Technol. 2015;68:50–55. doi: 10.1016/j.enzmictec.2014.10.005.
- Li Y, Lin Z, Huang C, Zhang Y, Wang Z, Tang Y, Chen T, Zhao X. Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab Eng. 2015;31:13–21. doi: 10.1016/j.ymben.2015.06.006.
- Fordjour E, Mensah EO, Hao Y, Yang Y, Liu X, Li Y, Liu CL, Bai Z. Toward improved terpenoids biosynthesis: strategies to enhance the capabilities of cell factories. Bioresour Bioprocess. Springer Science and Business Media Deutschland GmbH; 2022.
- Chen H, Li M, Liu C, Zhang H, Xian M, Liu H. Enhancement of the catalytic activity of Isopentenyl diphosphate isomerase (IDI) from Saccharomyces cerevisiae through random and site-directed mutagenesis. Microb Cell Fact. 2018;17:65. doi: 10.1186/s12934-018-0913-z.
- Wang F, Lv X, Xie W, Zhou P, Zhu Y, Yao Z, Yang C, Yang X, Ye L, Yu H. Combining Gal4p-mediated expression enhancement and directed evolution of isoprene synthase to improve isoprene production in Saccharomyces cerevisiae. Metab Eng. 2017;39:257–266. doi: 10.1016/j.ymben.2016.12.011.
- Leonard E, Ajikumar PK, Thayer K, Xiao W-H, Mo JD, Tidor B, Stephanopoulos G, Prather KLJ. Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proceedings of the National Academy of Sciences. 2010;107:13654–13659. doi: 10.1073/pnas.1006138107.
- Edgar S, Li F-S, Qiao K, Weng J-K, Stephanopoulos G. Engineering of Taxadiene Synthase for Improved Selectivity and Yield of a Key Taxol Biosynthetic Intermediate. ACS Synth Biol. 2017;6:201–205. doi: 10.1021/acssynbio.6b00206.
- Shimura K, Okada A, Okada K, Jikumaru Y, Ko K-W, Toyomasu T, Sassa T, Hasegawa M, Kodama O, Shibuya N, et al. Identification of a Biosynthetic Gene Cluster in Rice for Momilactones. Journal of Biological Chemistry. 2007;282:34013–34018. doi: 10.1074/jbc.M703344200.
- Wu Y, Hillwig ML, Wang Q, Peters RJ. Parsing a multifunctional biosynthetic gene cluster from rice: Biochemical characterization of CYP71Z6 & 7. FEBS Lett. 2011;585:3446–3451. doi: 10.1016/j.febslet.2011.09.038.
- Field B, Fiston-Lavier A-S, Kemen A, Geisler K, Quesneville H, Osbourn AE. Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proceedings of the National Academy of Sciences. 2011;108:16116–16121. doi: 10.1073/pnas.1109273108.
- Katerova Z, Todorova D, Tasheva K, Sergiev I. Influence of ultraviolet radiation on plant secondary metabolite production. Genet Plant Physiol. 2012;2:113–144.
- Thulasiram H V, Erickson HK, Poulter CD. Chimeras of Two Isoprenoid Synthases Catalyze All Four Coupling Reactions in Isoprenoid Biosynthesis. Science (1979). 2007;316:73–76. doi: 10.1126/science.1137786.
- Abdallah II, Quax WJ. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sciences. 2017;3:81–98. doi: 10.18502/kls.v3i5.981.
- Yadav N, Yadav R, Goyal A. Chemistry of Terpenoids Nita. Int J Pharm Sci Rev Res. 2009;27:272–278. doi: 10.1016/j.joms.2009.06.016. Cited: in: : PMID: 19835745.
- Rubulotta G, Quadrelli EA. Chapter 11 - Terpenes: A Valuable Family of Compounds for the Production of Fine Chemicals. In: Albonetti S, Perathoner S, Quadrelli EA, editors. Horizons in Sustainable Industrial Chemistry and Catalysis. Elsevier; 2019. p. 215–229.
- Hanson JR. Diterpenoids. Nat Prod Rep. 2009;26:1156–1171. doi: 10.1039/b807311m.
- Ludwiczuk A, Skalicka-Woźniak K, Georgiev MI. Chapter 11 - Terpenoids. In: Badal S, Delgoda R, editors. Pharmacognosy. Boston: Academic Press; 2017. p. 233–266.
- Nagarajan J, Ramanan RN, Raghunandan ME, Galanakis CM, Krishnamurthy NP. Chapter 8 - Carotenoids. In: Galanakis CM, editor. Nutraceutical and Functional Food Components. Academic Press; 2017. p. 259–296.
- Pegu F. Pharmacological activities of bioactive compounds isolated from Acacia pennata (L) Willd: A comprehensive update and application of in-silico techniques for repurposing. Sciences of Phytochemistry. 2022;1:1–12. doi: 10.58920/sciphy01010001.
- Kamel HN, Slattery M. Terpenoids of Sinularia: Chemistry and biomedical applications. Pharm Biol. 2005;43:253–269. doi: 10.1080/13880200590928852.
- Caputi L, Aprea E. Use of Terpenoids as Natural Flavouring Compounds in Food Industry. Recent Patents on Food, Nutrition & Agriculturee. 2012;3:9–16. doi: 10.2174/2212798411103010009.
- Fehr C, Magpantay I, Arpagaus J, Marquet X, Vuagnoux M. Enantioselective synthesis of (-)-β-Santalol by a copper-catalyzed enynol cyclization-fragmentation reaction. Angewandte Chemie - International Edition. 2009;48:7221–7223. doi: 10.1002/anie.200903449.
- Tolstikova TG, Sorokina I V., Tolstikov GA, Tolstikov AG, Flekhter OB. Biological activity and pharmacological prospects of lupane terpenoids: II. Semisynthetic lupane derivatives. Russ J Bioorg Chem. 2006;32:261–276. doi: 10.1134/s1068162006030083.
- Gross H, König GM. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochemistry Reviews. 2006;5:115–141. doi: 10.1007/s11101-005-5464-3.
- Jiang Z-H, Wang J-R, Li M, Liu Z-Q, Chau K-Y, Zhao C, Liu L. Hemiterpene Glucosides with Anti-Platelet Aggregation Activities from Ilex pubescens. J Nat Prod. 2005;68:397–399. doi: 10.1021/np049735y.
- Singh B, Sharma RA. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015;5:129–151. doi: 10.1007/s13205-014-0220-2.
- Vuorinen T, Reddy GVP, Nerg AM, Holopainen JK. Monoterpene and herbivore-induced emissions from cabbage plants grown at elevated atmospheric CO2 concentration. Atmos Environ. 2004;38:675–682. doi: 10.1016/j.atmosenv.2003.10.029.
- Litvak ME, Monson RK. Patterns of induced and constitutive monoterpene production in conifer needles in relation to insect herbivory. Oecologia. 1998;114:531–540. doi: 10.1007/s004420050477.
- Carpinella MC, Defago MT, Valladares G, Palacios SM. Antifeedant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. J Agric Food Chem. 2003;51:369–374. doi: 10.1021/jf025811w.
- Caputi L, Aprea E. Use of Terpenoids as Natural Flavouring Compounds in Food Industry. Recent Patents on Food, Nutrition & Agriculturee. 2012;3:9–16. doi: 10.2174/2212798411103010009.
- Chauhan R, Ruby KM, Dwivedi J. Secondary metabolites found in Bergenia species: A compendious review. Int J Pharm Pharm Sci. 2013;5:9–16.
- Kehrl W, Sonnemann U, Dethlefsen U. Therapy for Acute Nonpurulent Rhinosinusitis with Cineole: Results of a Double-Blind, Randomized, Placebo-Controlled Trial. Laryngoscope. 2004;114:738–742. doi: 10.1097/00005537-200404000-00027.
- Sarkic A, Stappen I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics. 2018;5:11. doi: 10.3390/cosmetics5010011.
- Carmona M, Zalacain A, Salinas MR, Alonso GL. Generation of Saffron Volatiles by Thermal Carotenoid Degradation. J Agric Food Chem. 2006;54:6825–6834. doi: 10.1021/jf0612326.
- Crowell PL, Ayoubi AS, Burke YD. Antitumorigenic Effects of Limonene and Perillyl Alcohol Against Pancreatic and Breast Cancer. Dietary Phytochemicals in Cancer Prevention and Treatment. Boston, MA: Springer US; 1996. p. 131–136.
- Crowell PL. Biochemistry and Physiology Prevention and Therapy of Cancer by Dietary Monoterpenes 1. Symposium on Phytochemicals: Biochemistry and Physiology. 1999;775–778.
- Crowell PL, Kennan WS, Haag JD, Ahmad S, Vedejs E, Gould MN. Chemoprevention of mammary carcinogenesis by hydroxylated derivatives of d-limonene. Carcinogenesis. 1992;13:1261-. doi: 10.1093/carcin/13.7.1261.
- Karapinar M, Esen Aktuǧ Ş. Inhibition of foodborne pathogens by thymol, eugenol, menthol and anethole. Int J Food Microbiol. 1987;4:161–166. doi: 10.1016/0168-1605(87)90023-7.
- Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. Cited: in: : PMID: 9218354.
- Brocksom TJ, Oliveira KT de, DesiderÃ!` AL. The Chemistry of the Sesquiterpene Alkaloids. J Braz Chem Soc. 2017;28:933–942.
- Gertsch J, Leonti M, Raduner S, Racz I, Chen J-Z, Xie X-Q, Altmann K-H, Karsak M, Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105.
- González-Coloma A, Valencia F, Martín N, Hoffmann JJ, Hutter L, Marco JA, Reina M. Silphinene sesquiterpenes as model insect antifeedants. J Chem Ecol. 2002;28:117–129. doi: 10.1023/A:1013566919874.
- Smith ECJ, Williamson EM, Wareham N, Kaatz GW, Gibbons S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry. 2007;68:210–217. doi: 10.1016/j.phytochem.2006.10.001.
- Lebwohl M, Sohn A. Ingenol mebutate (ingenol 3-angelate, PEP005): focus on its uses in the treatment of nonmelanoma skin cancer. Expert Rev Dermatol. 2012;7:121–128. doi: 10.1586/edm.12.13.
- Gauvin-Bialecki A, Aknin M, Smadja J. 24-O-ethylmanoalide, a manoalide-related sesterterpene from the marine sponge Luffariella cf. variabilis. Molecules. 2008;13:3184–3191. doi: 10.3390/molecules13123184.
- Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CWW, Fong HHS, Kinghorn AD, Brown DM, et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:80–84. doi: 10.1038/nm1095-1046.
- Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14:885–890. doi: 10.1016/j.drudis.2009.05.015.
- McChesney JD, Venkataraman SK, Henri JT. Plant natural products: Back to the future or into extinction? Phytochemistry. 2007;68:2015–2022. doi: 10.1016/j.phytochem.2007.04.032.
- Lee KH. Current Developments in the Discovery and Design of New Drug Candidates from Plant Natural Product Leads. J Nat Prod. 2004;67:273–283. doi: 10.1021/np030373o.
- Rajab M. In Silico Structure-Activity Study of Selected Triterpenoids as Potential Inhibitors of Mycolic Acid Transporter of Mycobacterial MmpL3 Receptor Protein. Sciences of Phytochemistry. 2024;3:82–90. doi: 10.58920/sciphy0302240.
- Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:1–16. doi: 10.3390/molecules21050559.
- Rates SMK. Plants as source of drugs. Toxicon. 2001. p. 603–613.
- Ríos JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005. p. 80–84.
- Juthy N, Islam G, Zehad A, Zannah S. Antidiabetic and Antihyperlipidemic Activity of Ethanolic Extract of Mentha viridis in Alloxan Induced Diabetic Rats. Sciences of Pharmacy. 2024;3:167–176. doi: 10.58920/sciphar0303258.
- Erhabor A, Erhabor O. Comparative Analysis of Phytochemical Composition of Aqueous Extracts from Azadirachta indica and Vernonia amygdalina. Sciences of Phytochemistry. 2024;3:91–97. doi: 10.58920/sciphy0302270.
- Barman D, Dey N, Sen S, Kakoti B, Vanlalhriatpuii C. Neuromodulatory Effect of Plant Metabolites. Sciences of Phytochemistry. 2022;1:41–59. doi: 10.58920/sciphy01010047.
- Kao T-C, Shyu M-H, Yen G-C. Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid Inhibit Inflammation via PI3K/Akt/GSK3β Signaling and Glucocorticoid Receptor Activation. J Agric Food Chem. 2010;58:8623–8629. doi: 10.1021/jf101841r.
- Abdelmouleh M, Boufi S, Belgacem MN, Dufresne A. Short natural-fibre reinforced polyethylene and natural rubber composites: Effect of silane coupling agents and fibres loading. Compos Sci Technol. 2007;67:1627–1639. doi: https://doi.org/10.1016/j.compscitech.2006.07.003.
- Wijayanti R, Susmayanti W, Meliana D, Fauziyah A, Anjeline A, Putri D, Ulya F. Antibacterial and Antifungal Activities of Pandanus amaryllifolius Leaf Extract, Fractions, and Isolate and Their Role in Anti-Dandruff Shampoo Optimization. Sciences of Pharmacy. 2025;4:51–65. doi: 10.58920/sciphar0402299.
- Ojo O, Mphahlele MP, Mmutlane EM, Ndinteh DT. Antimicrobial Activity of Triterpenoid and Steroidal Cinnamates from Vitellaria paradoxa. Phytochemistry. 2025;4:26. doi: 10.58920/sciphy0401293.
- Dahiru M, Oni A, Danga J, Alhaji A, Jonah F, Hauwa A, Muhammad Z. An In Vitro Assessment of the Antioxidant Activity of Detarium microcarpum Guill. & Perr. Fabaceae. Sciences of Phytochemistry. 2024;3:114–122. doi: 10.58920/sciphy0302267.
- Haldar S, Phapale PB, Kolet SP, Thulasiram H V. Expedient preparative isolation, quantification and characterization of limonoids from Neem fruits. Analytical Methods. 2013;5:5386–5391. doi: 10.1039/c3ay41136b.
- Haldar S, Mulani FA, Aarthy T, Dandekar DS, Thulasiram H V. Expedient preparative isolation and tandem mass spectrometric characterization of C-seco triterpenoids from Neem oil. J Chromatogr A. 2014;1366:1–14. doi: 10.1016/j.chroma.2014.09.006.
- Desmiaty Y, Sandhiutami NM, Fahleni F, Griselda A, Apriliana A. Enhanced Ability of Agarwood Leaves (Aquilaria malaccensis Lam.) Ointment as Wound Healing to Heal Second-Degree Burns in Rats. Sciences of Pharmacy. 2024;3:51–60. doi: 10.58920/sciphar0301214.
- Soyata A, Khoirunnisa K, Wahab S. Larvicidal Activity of Red Betel Leaves (Piper ornatum) Ethanolic Extract Against Mosquito Larvae. Sciences of Pharmacy. 2024;3:107–111. doi: 10.58920/sciphar0302213.
- Sarker SD, Latif Z, Gray AI. Natural Product Isolation. In: Sarker SD, Latif Z, Gray AI, editors. Natural Products Isolation. Totowa, NJ: Humana Press; 2005. p. 1–25.
- Luque De Castro MD, Jiménez-Carmona MM, Fernández-Pérez V. Towards more rational techniques for the isolation of valuable essential oils from plants. TrAC - Trends in Analytical Chemistry. 1999;18:708–716. doi: 10.1016/S0165-9936(99)00177-6.
- Abba M, Usman S, Ahmad M, Tahir A, Umar A. Exploring the Antiepileptic Potential of Amaranthus spinosus: An Experimental Study in Albino Mice. Sciences of Pharmacy. 2023;2:106–114. doi: 10.58920/sciphar02030106.
- Owolabi T, Amodu E, Danga J. Quality Control of Herbal Drug (Paxherbal Bitter Tea) Via Thin-Layer Chromatography and Phytoconstituent Analysis. Sciences of Pharmacy. 2023;2:115–121. doi: 10.58920/sciphar02030115.
- Guo W, Liu W, Chen G, Hong S, Qian C, Xie N, Yang X, Sun Y, Xu Q. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int Immunopharmacol. 2012;14:613–619. doi: 10.1016/j.intimp.2012.09.002.
- Shahzad Y, Shah SNH, Ansari MT, Riaz R, Safdar A, Hussain T, Malik M. Effects of drug-polymer dispersions on solubility and in vitro diffusion of artemisinin across a polydimethylsiloxane membrane. Chinese Science Bulletin. 2012;57:1685–1692. doi: 10.1007/s11434-012-5094-2.
- Singh PK, Wani K, Kaul-Ghanekar R, Prabhune A, Ogale S. From micron to nano-curcumin by sophorolipid co-processing: Highly enhanced bioavailability, fluorescence, and anti-cancer efficacy. RSC Adv. 2014;4:60334–60341. doi: 10.1039/c4ra07300b.
- Daramwar PP, Srivastava PL, Kolet SP, Thulasiram H V. Biocatalyst mediated regio- and stereo-selective hydroxylation and epoxidation of (Z)-α-santalol. Org Biomol Chem. 2014;12:1048–1051. doi: 10.1039/c3ob42174k.
- Kolet SP, Niloferjahan S, Haldar S, Gonnade R, Thulasiram H V. Biocatalyst mediated production of 6β,11α-dihydroxy derivatives of 4-ene-3-one steroids. Steroids. 2013;78:1152–1158. doi: 10.1016/j.steroids.2013.08.004.
- Haldar S, Kolet SP, Thulasiram H V. Biocatalysis: Fungi mediated novel and selective 12β- or 17β-hydroxylation on the basic limonoid skeleton. Green Chemistry. 2013;15:1311–1317. doi: 10.1039/c3gc40193f.
- Kiani BH, Kayani WK, Khayam AU, Dilshad E, Ismail H, Mirza B. Artemisinin and its derivatives: a promising cancer therapy. Mol Biol Rep. 2020;47:6321–6336. doi: 10.1007/s11033-020-05669-z.
- Sousa-Pimenta M, Estevinho LM, Szopa A, Basit M, Khan K, Armaghan M, Ibrayeva M, Sönmez Gürer E, Calina D, Hano C, et al. Chemotherapeutic properties and side-effects associated with the clinical practice of terpene alkaloids: paclitaxel, docetaxel, and cabazitaxel. Front Pharmacol. 2023;14. doi: 10.3389/fphar.2023.1157306.
- Vijayalakshmi V, Gokulakrishnan J, Prabhu V, Rahul S, Gopinath M. Systematic review of the therapeutic potential of medicinal herbs: insights into their biological applications and futuristic innovations. Proceedings of the Indian National Science Academy. 2025; doi: 10.1007/s43538-025-00477-0.
- Farco JA, Grundmann O. Menthol--pharmacology of an important naturally medicinal “cool”. Mini Rev Med Chem. 2013;1:124–131. Cited: in: : PMID: 23061635.
- Liu B. Functional Control of Cold- and Menthol-Sensitive TRPM8 Ion Channels by Phosphatidylinositol 4,5-Bisphosphate. Journal of Neuroscience. 2005;25:1674–1681. doi: 10.1523/jneurosci.3632-04.2005.
- Guentert M. The flavour and fragrance industry-past, present, and future. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. 2007.
- Sylvestre M, Nagau F, Legault J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L . from Guadeloupe. 2006;103:99–102. doi: 10.1016/j.jep.2005.07.011.
- Uronnachi E, Okpalaku O, Ikeotuonye C. Lemon Oil Enhances the Anti-Rheumatic Activity of Woody Essential Oils in Formaldehyde-Induced Arthritis in Wistar Rats. Sciences of Phytochemistry. 2024;3:44–53. doi: 10.58920/sciphy0301228.
- Butnariu M. Plants as Source of Essential Oils and Perfumery Applications. Bioprospecting of Plant Biodiversity for Industrial Molecules. Wiley; 2021. p. 261–292.
- Nwonuma CO, Omoniwa BP, Elleke TE, Aladele P, Ogundipe OE. The modes of action of biopesticidal compounds in insect control. Int J Trop Insect Sci. 2025;45:513–523. doi: 10.1007/s42690-025-01479-7.
- Mischko W, Hirte M, Roehrer S, Engelhardt H, Mehlmer N, Minceva M, Brück T. Modular biomanufacturing for a sustainable production of terpenoid-based insect deterrents. Green Chemistry. 2018;20:2637–2650. doi: 10.1039/C8GC00434J.
- Walkling CJ, Doppalapudi KR, Zhang DD, Harvey BG. Renewable Diesel Fuel Prepared by the Isomerization and Hydrogenation of Biosynthetic Farnesene. Energy & Fuels. 2025;39:8495–8500. doi: 10.1021/acs.energyfuels.4c05237.
- Caputi L, Aprea E. Use of Terpenoids as Natural Flavouring Compounds in Food Industry. Recent Patents on Food, Nutrition & Agriculturee. 2011;3:9–16. doi: 10.2174/2212798411103010009.
- Mittu B, Chaubey N, Singh M, Begum Z, Renubala, Neha. Cosmeceutical applications of terpenes and terpenoids. Specialized Plant Metabolites as Cosmeceuticals. Elsevier; 2024. p. 25–41.
- Alamgir ANM. Secondary Metabolites: Secondary Metabolic Products Consisting of C and H; C, H, and O; N, S, and P Elements; and O/N Heterocycles. 2018. p. 165–309.
- Sahu P, Bhowmick AK, Kali G. Terpene Based Elastomers: Synthesis, Properties, and Applications. Processes. 2020;8:553. doi: 10.3390/pr8050553.
- Sánchez-Velandia JE, Gallego-Villada LA, Mäki-Arvela P, Sidorenko A, Yu. Murzin D. Upgrading biomass to high-added value chemicals: synthesis of monoterpenes-based compounds using catalytic green chemical pathways. Catalysis Reviews. 2025;67:371–496. doi: 10.1080/01614940.2024.2329553.
- Zhang C, Hong K. Production of Terpenoids by Synthetic Biology Approaches. Front Bioeng Biotechnol. 2020;8. doi: 10.3389/fbioe.2020.00347.
- Dai J, Chen M, Xu D, Li H, Qiao Y, Ke X, Ci T. Self-Assembly Delivery System Based on Small-Molecule Camptothecin Prodrug for Treatment of Colorectal Carcinoma. Nanomedicine. 2021;16:355–372. doi: 10.2217/nnm-2020-0453.
- Nurrachmat Mulianto, Nurul Hidayati. Menthol-Camphor-Thymol-Eucalyptus Compound as a Novel Adjuvant Therapy in Fluconazole-Treated Candida parapsilosis Onychomycosis: A Case Report. Bioscientia Medicina : Journal of Biomedicine and Translational Research. 2025;9:8220–8231. doi: 10.37275/bsm.v9i8.1348.

 ETFLIN
Notification
ETFLIN
Notification







