Pharmacological Activities of Bioactive Compounds Isolated from Acacia pennata (l) Willd: A Comprehensive Update and Application of In Silico Techniques for Repurposing
by Farida Pegu ★
Academic editor: James H. Zothantluanga
Sciences of Phytochemistry 1(1): 1-10 (2022); https://doi.org/10.58920/sciphy01010001
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
14 May 2022
25 Jun 2022
25 Jun 2022
26 Jun 2022
Abstract: Bioactive compounds (BACs) are naturally occurring compounds with pharmacological activities. BACs isolated from plants have significantly contributed to modern medicine. Multiple studies had reported the isolation of BACs with diverse pharmacological activities from Acacia pennata (L.) Willd. This review aims to compile all the available data on the pharmacological activities of the BACs that had been isolated from A. pennata. An online literature survey was carried out on academic databases namely Scopus, Science Direct, PubMed, and Google Scholar. Keywords such as ‘Acacia pennata’, ‘isolated compound’, and ‘pharmacological activity’ were used, either alone or in combination. A total of 52 articles published between the year 1980 to 2020 that contained relevant information on A. pennata were identified and collected. To date, a total of 29 compounds had been isolated from A. pennata. The compounds isolated from A. pennata belonged to secondary metabolites namely triterpenoid ketone, ceramide, alkaloid, saponin, flavonoid-glycoside, and terpenoid. A total of 22 BACs had been evaluated for biological activities such as anti-alzheimer, anti-inflammatory, antioxidant, anti-diabetic, anti-obesity, anti-viral, anti-nociceptive, and anti-cancer activities. The pharmacological activities of 7 compounds isolated from A. pennata remained unexplored. A total of 14 compounds that had been isolated from A. pennata were also reported to be isolated from other plants. This comprehensive review provides an update on all the pharmacological works that had been carried out on the isolated BACs of A. pennata to date. In silico techniques may be applied to repurpose the isolated BACs of A. pennata prior to wet lab studies.
Keywords: Acacia pennataBioactive compoundsEthnopharmacologyPhytochemistryIn-silico
Introduction
Bioactive compounds (BACs) are naturally occurring substances with pharmacological activity (1, 2). Some BACs isolated from plants such as quinine, aspirin, digoxin, reserpine, vinblastine, atropine, colchicine, artemisinin, ephedrine, morphine, pilocarpine, physostigmine, taxol, quinidine, tubocurarine, and vincristine are some examples of BACs that are currently used as pharmaceutical drugs in the modern system of medicine (3). Notably, 1/5th of all the identified plants are employed in pharmaceutical studies and positively impact the healthcare system (4). The medicinal value of a plant is attributed to the presence of BACs (3). From this, it can be deciphered that the healthcare system benefits from BACs capable of eliciting the desired therapeutic actions.
The nutritive values, vegetative values, medicinal properties, and pharmacological activities of Acacia pennata (L.) Willd. is well documented (5). Studies have also reported the presence of different classes of BACs as well as the isolation of multiple BACs from A. pennata (5, 6). A. pennata is distributed in India, Myanmar, Bangladesh, Bhutan, Sri Lanka, Thailand, Vietnam, and Southwest China (5, 7). The edible parts are consumed as a vegetable in India (in Mizoram, Nagaland, and Karnataka) and Thailand. The tribe of Dimasa and Karbi utilize A. pennata to prepare a local tribal wine and rice beer, respectively. Studies have also reported the presence of carbohydrates, fats, proteins, free amino acids, fibre, sodium, calcium, potassium, magnesium, zinc, iron, nitrogen, phosphorus, copper, manganese, selenium, vitamin A, vitamin B1, vitamin B2, vitamin B3 and vitamin C in A. pennata (5).
A. pennata is traditionally indicated for fever, diarrhea, bronchitis, burning urine, indigestion, skin burn, bleeding gums, a disorder of the blood, headache, haemorrhoids, eruption, oculopathy, asthma, stomach ache, strengthening of bone, wound healing, removing fowl’s bone in the throat, toothache, snakebite, cholera, treating spasm, biliousness, dysentery, body pain, and flatulence. Studies have reported the antioxidant, anti-inflammatory, anti-herpes simplex virus, antidiabetic, pupicidal, larvicidal, anti-nociceptive, anticancer, anti-fungal, anthelmintic, anti-Alzheimer, anti-lice, anti-HIV activity, and anti-hyperlipidaemic activity of A. pennata (5, 6).
A. pennata contained different classes of plant secondary metabolites like flavonoids, glycosides, phenols, phytosterols, saponins, and terpenoids (5, 6). Interestingly, flavonoids, glycosides, phenols, phytosterols, saponins, and terpenoids were reported to exhibit different pharmacological activities (8-13). Thus, it is safe to hypothesize that the presence of secondary metabolites may be the reason for the traditional use of A. pennata to treat 25 different health ailments.
Despite the available works which appraise the value of A. pennata as a potent medicinal plant, there is still no systematic discussion on the pharmacological activities of the compounds that had been isolated from A. pennata. Moreover, the status of research on the BACs separated from A. pennata is unknown. Therefore, further developments may be hindered due to the lack of a comprehensive work on the pharmacological activities of the BACs isolated from A. pennata. Also, there is still no attempt to identify alternate natural sources for similar compounds isolated from A. pennata. This justifies the necessity for an up-to-date review on the concerned topic. Therefore, the present work aims to provide a comprehensive update on the pharmacological profile of the BACs isolated from A. pennata.
Methodology
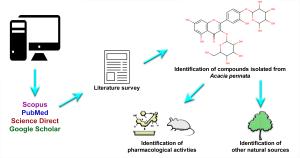
An online literature survey was carried out on databases like Scopus, PubMed, Science Direct, and Google Scholar. Whenever appropriate, the PubChem database was also referred to. Keywords such as ‘Acacia pennata’, ‘isolated compound’, and ‘pharmacological activity’ were used alone or in combination to search relevant articles. A total of 52 articles published between 1980 and 2020 were collected as they contained significant information to our satisfaction. The chemical class of each isolated compound was identified from the collected articles or the PubChem database. Other phytochemicals that were identified as present in A. pennata using chromatographic-spectroscopic techniques without any information on their isolation were not included in the review. To prevent any presentation of false information on the research gaps concerning the pharmacological activity of the compounds isolated from A. pennata, the pharmacological activities of all the similar compounds isolated from other plants were also identified and included in the review. The chemical structures were drawn with the Marvin Sketch v20.10 software. The correctness of the chemical structures was checked using the ‘Structure checker’ add-in of the Marvin Sketch software. The graphical abstract and figures were prepared with Adobe Photoshop CC 2017.
Pharmacological Activities of the BACs Isolated from A. pennata
To date, a total of 29 phytocompounds (Figure 1) have been isolated from the twigs, stems, aerial parts, and leaves of A. pennata. Of these, 22 BACs isolated from A. pennata or similar BACs isolated from other plants had been investigated for at least one pharmacological activity. However, the pharmacological activities of a terpenoid isolated from the leaves, namely labdanolic acid (C1) along with the flavonoid glycosides isolated from the aerial parts such as koaburanin (C2); 5,7-dihydroxyflavone 7-O-β-D-glucopyranosyl-8-C-β-boivinopyranoside (C3); 5,7-dihydroxyflavone 6-C-β-boivinopyranosyl-7-O-β-D-glucopyranoside (C4); (2R)-4’,7-dihydroxyflavan-(4aà8)-(2R,3S)-3,5,7-trihdyroxyflavan-3”-O-α-L-rhamnopyranoside (C5); (2S)-5,7-dihydroxyflavan-7-O-β-D-glucopyranoside-(4aà8)-epiafzelechin-3-O-gallate (C6) and (2R, 3S)-3,5,7-trihdyroxyflavan-3-O-α-L-rhamnopyranoside (C7) are still not investigated for any pharmacological activity (14, 15).
Quercetin 4’-O-α-L-rhamnopyranosyl-3-O-β-D-allopyranoside (C8) is a flavonoid-glycoside reported to be isolated from the leaves of A. pennata. C8 inhibited cyclooxygenase (COX)-1 (80.4 % inhibition at 10-4 g/ml; IC50 = 11.6 µg/ml) and COX-2 (12.6 % inhibition at 10-4 g/ml) in a COX-1/COX-2 catalysed prostaglandin biosynthesis assay (CPBA) (16).Apigenin 6-C-(2”-O-(E)-feruloyl-β-D-glucopyranosyl)-8-C-β-glucopyranoside (C9) is a flavonoid-glycoside reported to be isolated from the leaves of A. pennata. C9 inhibited COX-2 (8.6 % inhibition at 10-4 g/ml) in a COX-1/COX-2-CPBA (16).Isorhamnetin 3-O-α-L-rhamnopyranoside (C10) is a flavonoid-glycoside reported to be isolated from the leaves of A. pennata. C10 inhibited COX-1 (74.0 % inhibition at 10-4 g/ml; IC50 = 24.4 µg/ml) in a COX-1/COX-2-CPBA (16).Kaempferol 3-O-α-L-rhamnopyranosyl-(1à4)-β-D-glucopyranoside (C11) is a flavonoid-glycoside reported to be isolated from the leaves of A. pennata. C11 inhibited COX-1 (49.4 % inhibition at 10-4 g/ml; IC50 = 157.8 µg/ml) and COX-2 (5.0 % inhibition at 10-4 g/ml) in a COX-1/COX-2-CPBA (16).
Isovitexin (C12) is a flavonoid-glycoside reported to be isolated from the leaves of A. pennata. C12 inhibited COX-1 (66.4 % inhibition at 10-4 g/ml; IC50 = 30.6 µg/ml) and COX-2 (7.4 % inhibition at 10-4 g/ml) in a COX-1/COX-2-CPBA (16). C12 inhibited the stem-like cells in hepatic carcinoma by regulating manganese superoxide dismutase and forkhead box protein M1 (17). C12 inhibited α-amylase, α-glucosidase, and the formation of advanced glycation end products with IC50 values of 0.2826, 0.0469, and 0.0252 mg/ml, respectively (18). C12 exerts anti-inflammatory activity against lipopolysaccharide (LPS) induced neuroinflammation in BV-2 cells and mouse primary microglia by increasing the expression of M2 microglial marker, suppressing the expression of M1 microglial marker, increasing the release of interleukin 10, and by activating the Ca2+ dependent protein kinase/AMP-activated protein kinase-PGC-1α signalling pathway (19). Another study reported that C12 inhibits the production of reactive oxygen species induced by fine airborne particles of particulate matter of fewer than 2.5 micrometres (20). C12 also inhibits 2,2-diphenyl-1-picrylhydrazyl (DPPH) (IC50 = 1.72 mg/ml), 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (IC50 = 0.94±0.01 mg/ml) and superoxide anion (IC50 = 0.18 mg/ml) free radicals. C12 increases CD133 and β-catenin (stem cell markers), indicating its potential to prevent skin damage. Moreover, C12 showed antioxidant and anti-inflammatory activity in LPS-induced acute lung injury, simulated in vitro in RAW 264.7 cells and in vivo in mice (21).
Taepeenin D (C13) is a terpenoid reported to be isolated from the leaves of A. pennata. C13 exhibits anticancer activity against human prostate (DU145) (IC50 = 3.4 µM) and pancreatic cancer cells (PANC1) (IC50 = 3.2 µM). Interestingly, C13 did not exhibit toxicity against normal cells. It reduces the tumor suppressor patched one protein (PTCH) and antiapoptotic B-cell lymphoma 2 (BCL-2) protein in a dose-dependent manner. C13 downregulates the expression of mRNA of PTCH in PANC1. This is suggestive of its inhibitory effect on the transcription of Hedgehog/glioma-associated oncogene (14). Another study reported that Taepeenin D inhibited LPS-induced nitric oxide (NO) and tumor necrosis factor (TNF)-α production in RAW 264.7 cell lines with IC50 values of 8.2 and 38.8 µM, respectively (22). (+)-drim-8-ene (C14) is a terpenoid reported to be isolated from the leaves of A. pennata. C14 exhibits anti-cancer activity against DU145 (IC50 = 23.2 µM) and PANC1 (IC50 = 15.1 µM). Interestingly, C14 remains non-toxic to normal cells. (+)-drim-8-ene was reported to reduce the level of the tumor suppressor PTCH and antiapoptotic BCL-2 protein in a dose-dependent manner (14).

Figure 1. Chemical structures of the compounds isolated from A. pennata.
8,15-labdanediol (C15) is a terpenoid reported to be isolated from the leaves of A. pennata (14). 8,15-labdanediol inhibited LPS-induced NO synthase and prostaglandin E2 production in a LPS treated RAW 264.7 macrophages cell line with IC50 values of 15±1.1 and 25±3.2 µM, respectively (23). Quercetin 3-O-β-D-glucopyranosyl-4-O-β- D-glucopyranoside (C16) is a flavonoid-glycoside reported to be isolated from the leaves of A. pennata. C16 showed anti-cancer activity against DU145 (IC50 = 30.0 µM) and PANC1 (IC50 = 26.6 µM). Interestingly, C16 remains non-toxic to normal cells. C16 reduces the PTCH and BCL-2 protein levels in a dose-dependent manner (14).
Tetracosane (C17) is a straight-chain alkane reported to be isolated from the twigs of A. pennata (24, 25). At 100 µM, C17 effectively inhibits the aggregation of β-amyloid (% inhibition = 65.0±1.8; IC50 = 0.4 µM). At 100 µg/ml, C17 weakly inhibits acetylcholinesterase (% inhibition = 14.8±0.7). At 1 mg/ml, C17 weakly inhibits DPPH free radicals (% inhibition = 5.8±1.4) (24). C17 also exhibits anti-cancer activity against HT-29 colon cancer cells, estrogen-dependent breast cancer (MDA-MB-231) cells, and gastric cancer cells (AGS) with IC50 values of 128.7, +250, and +250 μM respectively (26).
1-(heptyloxy)-octadecane (C18) is a straight-chain alkane reported to be isolated from the twigs of A. pennata (Lomarat et al. 2015; PubChem 2020b) (24, 27). At 100 µM, C18 effectively inhibits the aggregation of β-amyloid (% inhibition = 58.9±1.8; IC50 = 12.3 µM) (24). Methyl tridecanoate (C19) is a fatty acid methyl ester reported to be isolated from the twigs of A. pennata (24, 28). At 100 µM, C19 moderately inhibits the aggregation of β-amyloid (% inhibition = 32.2±2.7). At 100 µg/ml, C19 weakly inhibits acetylcholinesterase (% inhibition = 20.7±1.1) (24).
Arborinone (C20) is a triterpenoid ketone reported to be isolated from the twigs of A. pennata (24, 29). At 100 µM, C20 moderately inhibits the aggregation of β-amyloid (% inhibition = 47.8±1.6). At 1 mg/ml, C20 weakly inhibits DPPH free radicals (% inhibition = 5.5±0.3) (24). Confertamide A (C21) is a ceramide reported to be isolated from the twigs of A. pennata (2430). At 1 mg/ml, C21 weakly inhibits DPPH free radicals (% inhibition = 1.2±0.4) (24). 4-hydroxy-1-methyl-pyrrolidin-2-carboxylic acid (C22) is an alkaloid reported to be isolated from the twigs of A. pennata (24, 31). At 100 µM, C22 moderately inhibits the aggregation of β-amyloid (% inhibition = 32.1±6.0). At 100 µg/ml, C22 weakly inhibits acetylcholine esterase (% inhibition = 14.1±0.8). At 1 mg/ml, C22 weakly inhibits DPPH free radicals (% inhibition = 7.7±0.4) (24).
Quercetin-3-O-β-D-glucopyranoside (C23) is a flavonoid-glycoside reported to be isolated from the aerial parts of A. pennata (15). C23 showed in vitro (EC50 = 5.3 µM; EC90 = 9.3 µM) and in vivo (BALB/c or C57BL/6 mice model) inhibitory activity against the Ebola virus (32). C23 showed weak antimicrobial activity against various gram-positive bacteria, gram-negative bacteria, and fungi (minimum inhibitory concentration and IC50 value against different microbes ranged from 100 to >400 µg/ml and from 99.72 to 167.61 µg/ml, respectively). With IC50 values of 82.55 and 97.52 µg/ml, C23 showed antioxidant activities against DPPH free radicals and β-carotene bleaching respectively (33).
Quercetin-3-O-α-L-rhamnopyranoside (C24) is a flavonoid-glycoside isolated from the aerial parts of A. pennata (15). C24 showed strong inhibition of human recombinant aldose reductase in vitro (IC50 = 11.5±0.05). C24 significantly reduces sorbitol accumulation in the rat lens (34). C24 showed an immunomodulatory activity against the H1N1 virus (35, 36). C24 non-competitively inhibits the pancreatic lipase (IC50 = 100.56 µM) hinting at its anti-obesity potential (37). C24 showed significant antioxidant activity in the human umbilical vein endothelial cells model by increasing the activities of enzymatic antioxidants (superoxide dismutase and glutathione) and by inhibiting hydrogen peroxide (H2O2) induced apoptosis. C24 reduces the production of free radicals and deoxyribonucleic acid fragments mediated by H2O2 (38).
Chrysin-7-O-β-D-glucopyranoside (C25) is a flavonoid-glycoside reported to be isolated from the aerial parts of A. pennata (15). C25 showed weak antimicrobial activity against various gram-positive bacteria, gram-negative bacteria, and fungi (minimum inhibitory concentration and IC50 value against different microbes ranged from 150 to >400 µg/ml and from 109.27 to 293.67 µg/ml, respectively). With IC50 values of 102.35 and 140.48 µg/ml, C25 showed an antioxidant activity against DPPH free radicals and β-carotene bleaching, respectively (33). C25 exhibits hypotensive and diuretic activities. C25 increases the α-transcriptional action in MCF-7 cells. C25 inhibits the growth of Acinetobacter baumannii by 10 mm at 0.001 mg/ml. C25 inhibits α-glucosidase activity by 70% and 90% at 0.05 and 0.1 mg/ml respectively (39). A molecular docking simulation study showed the potential to inhibit nicotinamide phosphor ribosyl transferase in human colon cancer cells (40).
Kaempferol 3-O-α-L-rhamnopyranoside (C26) is a flavonoid-glycoside reported to be isolated from the aerial parts of A. pennata (15). C26 inhibits DPPH free radicals with an SC50 value of 12.45 µg/ml (41). Pinocembrin-7-O-β-D-glucopyranoside (C27) is a flavonoid-glycoside isolated from the aerial parts of A. pennata (15). C27 exhibits significant hepatoprotective activity in rats (42). 21β-O-((2E)-6-hydroxyl-2,6-dimethyl-2,7-octadienoyl) pitheduloside G (C28) is a saponin reported to be isolated from the stem of A. pennata. C28 was reported to inhibit the human immunodeficiency virus (HIV)-1 protease (PR) in vitro (IC50 = 2.0 ± 0.2 µM) (7). Pitheduloside G (C29) is a saponin reported to be isolated from the stem of A. pennata. C29 was reported to inhibit the HIV-1 PR in vitro (IC50 = 18 ± 0.5 µM) (7).
Alternate Natural Sources of BACs Isolated from A. pennata
Taepeenin D was also reported to be isolated from the roots of Caesalpinia mimosoides (22). Labdanolic acid was also reported to be isolated from Psiadia arguta leaves and Cistus palinhae. Other compounds isolated from P. arguta were tested for their antiplasmodial activity, but labdanolic acid was not tested for its antiplasmodial activity (43, 44). 8,15-labdanediol was also isolated from C. palinhae and Oxylobus glanduliferus (23, 44). Tetracosane was also reported to be isolated from the aerial parts of Acrostichum aureum L (26). Arborinone was also reported to be isolated from the powder coating of Lingnania chungii MCCLURE (29). Confertamide A was also reported to be isolated from Sinularia conferta (30). 4-hydroxy-1-methyl-pyrrolidin-2-carboxylic acid was also isolated from the leaves and stem of Toddalia aculeate (31).
Quercetin-3-O-β-D-glucopyranoside was also reported to be isolated from the leaves of Azadirachta indica. At 500 µg/disc, the ethyl acetate extract of A. indica showed a zone of inhibition ranging from 06–10 mm against various gram-positive bacteria, gram-negative bacteria, and fungi. Hexane, butanol, and ethyl acetate extract of A. indica showed cytotoxicity against Artemia salina (shrimp in simulated brine water) with IC50 values of 1.3, 10.2, and 0.61 µM, respectively. Even though Quercetin-3-O-β-D-glucopyranoside was reported to be isolated from A. indica, it was not explicitly investigated for its antimicrobial or anticancer activity (45). As other phytocompounds could also induce antimicrobial and anticancer activity, these activities were not considered for review.
Quercetin-3-O-β-D-glucopyranoside was also reported to be isolated from Halostachys caspica C. A. Mey aerial parts; leaves of Euphorbia heterophylla L. and leaves of Loranthus kaoi (Chao) Kiu (33, 46, 47). Quercetin-3-O-α-L-rhamnopyranoside was also reported to be isolated from Chamaecyparis obtuse leaves (34), Rapanea melanophloeos (L.) (35), Euphorbia heterophylla L. leaves (46), Polygonum aviculare L. (37), Bronowicka Ostra (a variety of hot pepper) (48), Lindera aggregata (Sims) Kosterm (38), and Mimosa pigra L. leaves (49).
Chrysin-7-O-β-D-glucopyranoside was also reported to be isolated from Calycotome villosa subsp. Intermedia flowers and leaves (50), Halostachys caspica C. A. Mey aerial parts (33), and Calicotome villosa stems (39). Kaempferol 3-O-α-L-rhamnopyranoside was also reported to be isolated from Raphanus raphanistrum L. aerial parts. The extract of R. raphanistrum was evaluated for in vitro cytotoxic activity. However, Kaempferol 3-O-α-L-rhamnopyranoside was not investigated explicitly for its cytotoxic activity (51). Thus, this activity was not included in the review. Kaempferol 3-O-α-L-rhamnopyranoside was also reported to be isolated from Chenopodium ambrosioides L. leaves (41) and Dennstaedtia scandens (BLUME) MOORE fronds (52).
Pinocembrin-7-O-β-D-glucopyranoside was also reported to be isolated from Penthorum chinense Pursh aerial parts (42), Viscum articulatum whole dried plants (53), leaves of Loranthus kaoi (Chao) Kiu (47) and Elytranthe parasitica (L.) Danser (EP) (54). Though pinocembrin-enriched fractions of E. parasitica showed potential anticancer activity, Pinocembrin-7-O-β-D-glucopyranoside was never specifically investigated for its anticancer activity (54). Thus, this activity was not included in the review. Pitheduloside G was also reported to be isolated from the seeds of Pithecellobium dulce (55).
In-silico Techniques for Repurposing BACs Isolated from A. pennata
In-silico techniques have been increasingly used in the field of pharmaceutical research. Computational approaches such as molecular docking, molecular dynamics (MD) simulations, calculation of binding free energies with molecular mechanics (MM)- generalized born surface area (GBSA)/Poisson Boltzmann surface area (PBSA) approaches are popularly used to study the binding affinity, molecular interactions, and molecular mechanisms of chemicals against drug targets (56, 57). Computational techniques can also be combined with in-vitro and in-vivo studies (58, 59). However, there should be some similarities in the models used for the in-silico and wet lab studies. For example, in the case of antidiabetic evaluation, α-amylase may be used for in-silico and in-vitro studies (58). Also, in the case of cerebroprotective studies of chemicals, biomarkers such as interleukins or tumor necrosis factors may be used for in-vivo and in-silico studies (59, 60). It is considered illogical to randomly apply in-silico models that do not correlate with in-vitro or in-vivo models.
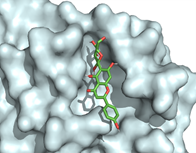
Figure 2. Binding pose of isovitexin at the active binding site of SARS-CoV-2 Mpro (reproduced with permission from Zothantluanga et al. 2022, http://dx.doi.org/10.1186/s43094-021-00348-7)
Drug repurposing is investigating a compound for other therapeutic purposes than what it was initially intended for (61). Researchers have used in-silico techniques to repurpose phytochemicals that are present in Indian spices as inhibitors of the main protease (Mpro) and papain-like protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (62). Food and Drug Administration-approved drugs were also repurposed for a popular drug target of Plasmodium falciparum, dihydrofolate reductase thymidylate synthase (PfDHFR-TS) (63). Molecular docking and MD simulations were used to screen phytocompounds for their potential application in treating cancer (64, 65). The phytochemicals in antiviral medicinal plants such as Baccaurea ramiflora and Bergenia ciliata have been studied with molecular docking, MD simulations, MM-GBSA calculations, and density functional theory studies to investigate their inhibitory potential against SARS-CoV-2 Mpro (66). Bioactive molecules of a traditional Ayurvedic herbal formulation were repurposed to inhibit SARS-CoV-2 Mpro (67). Many researchers are applying in-silico techniques to discover new molecules for therapeutic applications.
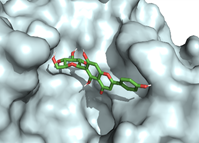
Figure 3. Binding pose of isovitexin at the active binding site of SARS-CoV-2 Mpro (reproduced with permission from Zothantluanga et al. 2021, http://dx.doi.org/10.1186/s43094-021-00348-7)
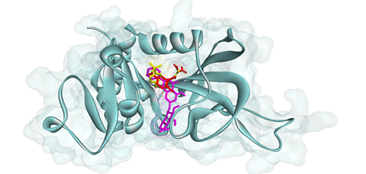
Figure 4. Binding pose of pinocembrin-7-O-β-D-glucopyranoside at the active binding site of PfDHFR-TS. RJ1 (yellow color) of the original co-crystallized complex, RJ1 (red color) re-docked with PyRx 0.8 tool, and C27 (purple color) docked with PyRx0.8 tool (reproduced with permission from Zothantluanga et al. 2022, http://dx.doi.org/10.33263/BRIAC124.48714887)
Of all the phytocompounds present in A. pennata, isovitexin was found as the most promising phytochemical, with the potential to inhibit the viral replication of SARS-CoV-2 as well as to prevent the cellular entry of SARS-CoV-2 by binding to active binding sites of SARS-CoV-2 Mpro (Figure 2) and furin (Figure 3). In-silico ADMET screening was executed, and it computed isovitexin as a safe, bioavailable, and non-toxic phytocompound (68). In another study, the flavonoid phytocompounds of A. pennata were studied for their potential antimalarial activity by targeting the PfDHFR-TS of P. falciparum. Molecular docking with two different virtual screening tools, in-silico ADMET screening and bioactivity prediction, revealed pinocembrin-7-O-β-D-glucopyranoside as a promising lead compound for inhibiting PfDHFR-TS (Figure 4) (69). These in-silico studies support the claim that computational techniques can be used to repurpose the isolated BACs of A. pennata for other health ailments. In-silico techniques can also be used to study the molecular interactions, hypothesize the molecular mechanisms, and determine the inhibitory potential of the isolated BACs against multiple drug targets.
Conclusions
A. pennata is a Southeast Asian medicinal plant with a diverse range of biologically active compounds. This explains the traditional use of A. pennata for 25 different health ailments. The biological activity of 7 phytocompounds that remains unexplored may be investigated in the future. In-silico techniques can be applied to investigate the potential activity of the 7 phytocompounds whose activity remained unexplored. Moreover, the 22 BACs may also be repurposed for other health ailments. Before wet-lab studies are carried out for repurposing, the potential activities of the 22 BACs may also be investigated with in-silico techniques. This comprehensive review provides an update on all the pharmacological works carried out on the isolated BACs of A. pennata to date. This review will benefit researchers working in the field of natural products.
Declarations
Acknowledgment
The author acknowledges and gives the warmest thanks to all seniors for their assistance and for their help in writing the manuscript. The author also thanks the faculty of the JB Institute of Pharmacy and a close friend for their continuous support. The author is grateful to all family members (especially parents) and expresses the highest gratitude to them.
Ethics Statement
Not applicable.
Data Availability
Not applicable.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Pathak, K.; Pathak, M. P.; Saikia, R.; Gogoi, U.; Sahariah, J. J.; Zothantluanga, J. H.; Samanta, A.; Das, A. Cancer Chemotherapy via Natural Bioactive Compounds. Curr. Drug Discov. Technol. 2022. https://doi.org/10.2174/1570163819666220331095744
- Angiolillo, L.; Del Nobile, M. A.; Conte, A. The Extraction of Bioactive Compounds from Food Residues Using Microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98.
- Paul, S.; Hmar, E. B. L.; Zothantluanga, J. H.; Sharma, H. K. Essential Oils: A Review on Their Salient Biological Activities and Major Delivery Strategies. Sci. Vis. 2020, 20, 54–71.
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542.
- Zothantluanga, J. H.; Bhat, H. R.; Shakya, A. A Systematic Review on the Nutraceutical Potential of Acacia Pennata (L.) Willd. Curr. Trends Pharm. Res. 2019, 6, 12–27.
- Zothantluanga, J. H.; Sailo, N.; Paul, A.; Shakya, A. Pharmacognostical Characterization and in Vitro Antioxidant Activity of Acacia Pennata (L.) Willd. Leaves: A Southeast Asian Medicinal Plant. Sci. Vis. 2020, 20, 16–28.
- Nguyen, V. D.; Nguyen, H. L. T.; Do, L. C.; Van Tuan, V.; Thuong, P. T.; Phan, T. N. A New Saponin with Anti-HIV-1 Protease Activity from Acacia Pennata. Nat. Prod. Commun. 2018, 13, 1934578X1801300.
- Agrawal, A. D. Pharmacological Activities of Flavonoids: A Review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398.
- Gálvez, M.; Martín-Cordero, C.; Ayuso, M. J. Pharmacological Activities of Phenylpropanoids Glycosides; 2006. 675–718.
- Sun, J.; Liu, J. N.; Fan, B.; Chen, X. N.; Pang, D. R.; Zheng, J.; Zhang, Q.; Zhao, Y. F.; Xiao, W.; Tu, P. F.; et al. Phenolic Constituents, Pharmacological Activities, Quality Control, and Metabolism of Dracaena Species: A Review. J. Ethnopharmacol. 2019, 244, 112138.
- Moghadasian, M. H. Pharmacological Properties of Plant Sterols. Life Sci. 2000, 67, 605–615.
- Lacaille-Dubois, M. A.; Wagner, H. A Review of the Biological and Pharmacological Activities of Saponins. Phytomedicine 1996, 2, 363–386.
- Wang, S. C.; Chen, Y.; Wang, Y. C.; Wang, W. J.; Yang, C. S.; Tsai, C. L.; Hou, M. H.; Chen, H. F.; Shen, Y. C.; Hung, M. C. Tannic Acid Suppresses SARS-CoV-2 as a Dual Inhibitor of the Viral Main Protease and the Cellular TMPRSS2 Protease. Am. J. Cancer Res. 2020, 10, 4538–4546.
- Rifai, Y.; Arai, M. A.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. Terpenoids and a Flavonoid Glycoside from Acacia Pennata Leaves as Hedgehog/GLI-Mediated Transcriptional Inhibitors. J. Nat. Prod. 2010, 73, 995–997.
- Kim, A.; Choi, J.; Htwe, K. M.; Chin, Y. W.; Kim, J.; Yoon, K. D. Flavonoid Glycosides from the Aerial Parts of Acacia Pennata in Myanmar. Phytochemistry 2015, 118, 17–22.
- Dongmo, A.; Miyamoto, T.; Yoshikawa, K.; Arihara, S.; Lacaille-Dubois, M. A. Flavonoids from Acacia Pennata and Their Cyclooxygenase (COX-1 and COX-2) Inhibitory Activities. Planta Med. 2007, 73, 1202–1207.
- Cao, X.; Liu, L.; Yuan, Q.; Li, X.; Cui, Y.; Ren, K.; Zou, C.; Chen, A.; Xu, C.; Qiu, Y.; et al. Isovitexin Reduces Carcinogenicity and Stemness in Hepatic Carcinoma Stem-like Cells by Modulating MnSOD and FoxM1. J. Exp. Clin. Cancer Res. 2019, 38, 264.
- Sae-tan, S.; Saeting, O. Anti-Diabetic Activities of Vitexin and Isovitexin from Mung Bean Soup. J Nutr Food Sci 2017, 7, 39.
- Liu, B.; Huang, B.; Hu, G.; He, D.; Li, Y.; Ran, X.; Du, J.; Fu, S.; Liu, D. Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKβ/AMPK-PGC-1α Signaling Axis. Front. Immunol. 2019. https://doi.org/10.3389/fimmu.2019.02650
- Chowjarean, V.; Prueksasit, T.; Joyjamras, K.; Chanvorachote, P. Isovitexin Increases Stem Cell Properties and Protects Against PM2.5 in Keratinocytes. In Vivo (Brooklyn). 2019, 33, 1833–1841.
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin Exerts Anti-Inflammatory and Antioxidant Activities on Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting MAPK and NF-ΚB and Activating HO-1/Nrf2 Pathways. Int. J. Biol. Sci. 2016, 12, 72–86.
- Yodsaoue, O. Chemical Constituents from the Roots of Caesalpinia Mimosoides and Caesalpinia Pulcherrima and Their Anti-Inflammatory Activity, Prince of Songkla University, 2012. Dissertation.
- Girón, N.; Pérez-Sacau, E.; López-Fontal, R.; Amaro-Luis, J. M.; Hortelano, S.; Estevez-Braun, A.; de las Heras, B. Evaluation of Labdane Derivatives as Potential Anti-Inflammatory Agents. Eur. J. Med. Chem. 2010, 45, 3155–3161.
- Lomarat, P.; Chancharunee, S.; Anantachoke, N.; Kitphati, W.; Sripha, K.; Bunyapraphatsara, N. Bioactivity-Guided Separation of the Active Compounds in Acacia Pennata Responsible for the Prevention of Alzheimer’s Disease. Nat. Prod. Commun. 2015, 10, 1431–1434.
- PubChem. Tetracosane. https://pubchem.ncbi.nlm.nih.gov/compound/Tetracosane. Accessed 14 June 2022.
- Uddin, S. J.; Grice, D.; Tiralongo, E. Evaluation of Cytotoxic Activity of Patriscabratine, Tetracosane and Various Flavonoids Isolated from the Bangladeshi Medicinal Plant Acrostichum Aureum. Pharm. Biol. 2012, 50, 1276–1280.
- PubChem. Octadecane. https://pubchem.ncbi.nlm.nih.gov/compound/octadecane. Accessed 14 June 2022.
- PubChem. Methyl tridecanoate. https://pubchem.ncbi.nlm.nih.gov/compound/methyl tridecanoate. Accessed 14 June 2022.
- Akihisa, T.; Yamamoto, K.; Tamura, T.; Kimura, Y.; Iida, T.; Nambara, T.; Chang, F. C. Triterpenoid Ketones from Lingnania Chungii MCCLURE: Arborinone, Friedelin and Glutinone. Chem. Pharm. Bull. 1992, 40, 789–791.
- Su, J. Y.; Kuang, Y. Y.; Zeng, L. M.; Li, H. New Tetracyclic Diterpenoid and New Ceramides from the Soft Coral Sinularia Conferta. J. Asian Nat. Prod. Res. 2005, 7, 107–113.
- Jain, S. C.; Pandey, M. K.; Upadhyay, R. K.; Kumar, R.; Hundal, G.; Hundal, M. S. Alkaloids from Toddalia Aculeata. Phytochemistry 2006, 67, 1005–1010.
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic Efficacy of Quercetin 3-β- O - d -Glucoside against Ebola Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188.
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys Caspica and Their Antimicrobial and Antioxidant Activities. Molecules 2010, 15, 7933–7945.
- Kim, S.; Kim, J.; Lee, Y.; Bae, Y.; Lim, S. Inhibitory Effect of Quercetin-3-O-α-L-Rhamnopyranoside from Chamaecyparis Obtuse on Aldose Reductase and Sorbitol Accumulation. Korean J Med. Crop Sci 2010, i, 305–310.
- Mehrbod, P.; Abdalla, M. A.; Fotouhi, F.; Heidarzadeh, M.; Aro, A. O.; Eloff, J. N.; McGaw, L. J.; Fasina, F. O. Immunomodulatory Properties of Quercetin-3-O-α-L-Rhamnopyranoside from Rapanea Melanophloeos against Influenza a Virus. BMC Complement. Altern. Med. 2018, 18, 184.
- Mehrbod, P.; Ebrahimi, S. N.; Fotouhi, F.; Eskandari, F.; Eloff, J. N.; McGaw, L. J.; Fasina, F. O. Experimental Validation and Computational Modeling of Anti-Influenza Effects of Quercetin-3-O-α-L-Rhamnopyranoside from Indigenous South African Medicinal Plant Rapanea Melanophloeos. BMC Complement. Altern. Med. 2019, 19, 346.
- Park, J. Y.; Kim, C. S.; Park, K. M.; Chang, P.-S. Inhibitory Characteristics of Flavonol-3-O-Glycosides from Polygonum Aviculare L. (Common Knotgrass) against Porcine Pancreatic Lipase. Sci. Rep. 2019, 9, 18080.
- Huang, S.-H.; Tseng, J.-C.; Lin, C.-Y.; Kuo, Y.-Y.; Wang, B.-J.; Kao, Y.-H.; Muller, C. J. F.; Joubert, E.; Chuu, C.-P. Rooibos Suppresses Proliferation of Castration-Resistant Prostate Cancer Cells via Inhibition of Akt Signaling. Phytomedicine 2019, 64, 153068.
- Alhage, J.; Elbitar, H.; Taha, S.; Guegan, J.-P.; Dassouki, Z.; Vives, T.; Benvegnu, T. Isolation of Bioactive Compounds from Calicotome Villosa Stems. Molecules 2018, 23, 851.
- Nganou, B.; Tane, P.; Nchiozem, A.; Selvaraj, J.; Selvaraj, A.; Nanjan, C. Identification of Human NMPrtase Inhibitors from Adenocarpus Mannii; an in-Silico Approach. J Pharm Sci Res 2017, 9, 95–99.
- Ghareeb, M.; Saad, A.; Abdou, A.; Refahy, L.; Ahmed, W. A New Kaempferol Glycoside with Antioxidant Activity from Chenopodium Ambrosioides Growing in Egypt. Orient. J. Chem. 2016, 32, 3053–3061.
- Guo, W. W.; Qiu, F.; Chen, X. Q.; Ba, Y. Y.; Wang, X.; Wu, X. In-Vivo Absorption of Pinocembrin-7-O-β-D-Glucoside in Rats and Its in-Vitro Biotransformation. Sci. Rep. 2016, 6, 29340.
- Mahadeo, K.; Herbette, G.; Grondin, I.; Jansen, O.; Kodja, H.; Soulange, J.; Jhaumeer-Laulloo, S.; Clerc, P.; Gauvin-Bialecki, A.; Frederich, M. Antiplasmodial Diterpenoids from Psiadia Arguta. J. Nat. Prod. 2019, 82, 1361–1366.
- Pascual Teresa, J. D.; Urones, J. G.; Marcos, I. S.; Núñez, L.; Basabe, P. Diterpenoids and Flavonoids from Cistus Palinhae. Phytochemistry 1983, 22, 2805–2808.
- Islam, M.; Al-Amin, M.; Siddiqi, M. M. A.; Akter, S.; Haque, M. M.; Sultana, N.; Chowdhury, A. S. Isolation of Quercetin-3-O-Beta-d-Glucopyranoside from the Leaves of Azadirachta Indica and Antimicrobial and Cytotoxic Screening of the Crude Extracts. Dhaka Univ. J. Sci. 2012, 60, 11–14.
- Tostes, J. B. D. F.; Da Silva, A. J. R.; Kuster, R. M. Isolation and Characterization of Polyphenols from Euphorbia Heterophylla L. (Euphorbiaceae) Leaves. Rev. Fitos 2019, 13, 49.
- Lin, J. H.; Lin, Y. T. Flavonoids from the Leaves of Loranthus Kaoi (Chao) Kiu. J. Food Drug Anal. 1999, 7, 9.
- Materska, M.; Perucka, I.; Stochmal, A.; Piacente, S.; Oleszek, W. Quantitative and Qualitative Determination of Flavonoids and Phenolic Acid Derivatives from Pericarp of Hot Pepper Fruit Cv. Bronowicka Ostra. Polish J Food Nutr Sci 2003, 53, 72–76.
- Okonkwo, C. J.; Njoku, O. U.; Okonkwo, T. J. N.; Afieroho, O. E.; Proksch, P. Two New Acylated Flavonol Glycosides from Mimosa Pigra L. Leaves Sub-Family Mimosoideae. Futur. J. Pharm. Sci. 2016, 2, 71–75.
- Antri, A.; Messouri, I.; Tlemçani, R.; Bouktaib, M.; El Alami, R.; El Bali, B.; Lachkar, M. Flavone Glycosides from Calycotome Villosa Subsp. Intermedia. Molecules 2004, 9, 568–573.
- Ibrahim, L.; Elkhateeb, A.; Marzouk, M.; Hussein, S.; Abdel-Hameed, E.; Kassem, M. Flavonoid Investigation, LC–ESI-MS Profile and Cytotoxic Activity of Raphanus Raphanistrum L. (Brassicaceae). J Chem Pharm Res 2016, 8, 786–793.
- Tanaka, N.; Nagase, S.; Wachi, K.; Murakami, T.; Saiki, Y.; Chen, C. Chemische Und Hemotaxonomische Untersuchungen von Filices. XXX. Chemische Untersuchungen Der Inhaltsstoffe von Dennstaedtia Scandens (BLUME) MOORE. Chem Pharm Bull 1980, 28, 2843–2845.
- Li, Y.; Zhao, Y. L.; Huang, N.; Zheng, Y. T.; Yang, Y. P.; Li, X. L. Two New Phenolic Glycosides from Viscum Articulatum. Molecules 2008, 13, 2500–2508.
- Kumar, N.; Shrungeswara, A. H.; Mallik, S. B.; Biswas, S.; Mathew, J.; Nandakumar, K.; Mathew, J.; Lobo, R. Pinocembrin-Enriched Fractions of Elytranthe Parasitica (L.) Danser Modulates Apoptotic and MAPK Cellular Signaling in HepG2 Cells. Anticancer. Agents Med. Chem. 2019, 18, 1563–1572.
- Nigam, S. K.; Gopal, M.; Uddin, R.; Yoshikawa, K.; Kawamoto, M.; Arihara, S. Pithedulosides A-G, Oleanane Glycosides from Pithecellobium Dulce. Phytochemistry 1997, 44, 1329–1334.
- Zothantluanga, J. H. Molecular Docking Simulation Studies, Toxicity Study, Bioactivity Prediction, and Structure-Activity Relationship Reveals Rutin as a Potential Inhibitor of SARS-CoV-2 3CL Pro. J. Sci. Res. 2021, 65, 96–104.
- Umar, A. K.; Zothantluanga, J. H.; Aswin, K.; Maulana, S.; Sulaiman Zubair, M.; Lalhlenmawia, H.; Rudrapal, M.; Chetia, D. Antiviral Phytocompounds “Ellagic Acid” and “(+)-Sesamin” of Bridelia Retusa Identified as Potential Inhibitors of SARS-CoV-2 3CL pro Using Extensive Molecular Docking, Molecular Dynamics Simulation Studies, Binding Free Energy Calculations, and Bioactivi. Struct. Chem. 2022. http://dx.doi.org/10.1007/s11224-022-01959-3
- Junejo, J. A.; Zaman, K.; Rudrapal, M.; Celik, I.; Attah, E. I. Antidiabetic Bioactive Compounds from Tetrastigma Angustifolia (Roxb.) Deb and Oxalis Debilis Kunth.: Validation of Ethnomedicinal Claim by in Vitro and in Silico Studies. South African J. Bot. 2021, 143, 164–175.
- Pasala, P. K.; Abbas Shaik, R.; Rudrapal, M.; Khan, J.; Alaidarous, M. A.; Jagdish Khairnar, S.; Bendale, A. R.; Naphade, V. D.; Kumar Sahoo, R.; Zothantluanga, J. H.; et al. Cerebroprotective Effect of Aloe Emodin: In Silico and in Vivo Studies. Saudi J. Biol. Sci. 2022, 29, 998–1005.
- Pasala, P. K.; Uppara, R. K.; Rudrapal, M.; Zothantluanga, J. H.; Umar, A. K. Silybin Phytosome Attenuates Cerebral Ischemia‐reperfusion Injury in Rats by Suppressing Oxidative Stress and Reducing Inflammatory Response: In Vivo and in Silico Approaches. J. Biochem. Mol. Toxicol. 2022. http://dx.doi.org/10.1002/jbt.23073
- Elfiky, A. A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp): A Molecular Docking Study. Life Sci. 2020, 253, 117592.
- Rudrapal, M.; Celik, I.; Chinnam, S.; Azam Ansari, M.; Khan, J.; Alghamdi, S.; Almehmadi, M.; Zothantluanga, J. H.; Khairnar, S. J. Phytocompounds as Potential Inhibitors of SARS-CoV-2 Mpro and PLpro through Computational Studies. Saudi J. Biol. Sci. 2022. http://dx.doi.org/10.1016/j.sjbs.2022.02.028
- Patowary, L.; Borthakur, M. S.; Zothantluanga, J. H.; Chetia, D. Repurposing of FDA Approved Drugs Having Structural Similarity to Artemisinin against PfDHFR-TS through Molecular Docking and Molecular Dynamics Simulation Studies. Curr. Trends Pharm. Res. 2021, 8, 14–34.
- Umar, A. K.; Zothantluanga, J. H. Structure-Based Virtual Screening and Molecular Dynamics of Quercetin and Its Natural Derivatives as Potent Oxidative Stress Modulators in ROS-Induced Cancer. Indones. J. Pharm. 2021, 3, 60.
- Umar, A. K.; Kelutur, F. J.; Zothantluanga, J. H. Flavonoid Compounds of Buah Merah (Pandanus Conoideus Lamk) as a Potent Oxidative Stress Modulator in ROS-Induced Cancer: In Silico Approach. Maj. Obat Tradis. 2021, 26, 221.
- Zothantluanga, J. H.; Abdalla, M.; Rudrapal, M.; Tian, Q.; Chetia, D.; Li, J. Computational Investigations for Identification of Bioactive Molecules from Baccaurea Ramiflora and Bergenia Ciliata as Inhibitors of SARS-CoV-2 M Pro. Polycycl. Aromat. Compd. 2022. http://dx.doi.org/10.1080/10406638.2022.2046613
- Rudrapal, M.; Celik, I.; Khan, J.; Ansari, M. A.; Alarousy, R. M. I. I.; Yadav, R.; Sharma, T.; Tallei, T. E.; Pasala, P. K.; Sahoo, R. K.; et al. Identification of Bioactive Molecules from Triphala (Ayurvedic Herbal Formulation) as Potential Inhibitors of SARS-CoV-2 Main Protease (Mpro) through Computational Investigations. J. King Saud Univ. - Sci. 2022, 101826.
- Zothantluanga, J. H.; Gogoi, N.; Shakya, A.; Chetia, D.; Lalthanzara, H. Computational Guided Identification of Potential Leads from Acacia Pennata (L.) Willd. as Inhibitors for Cellular Entry and Viral Replication of SARS-CoV-2. Futur. J. Pharm. Sci. 2021, 7, 201.
- Zothantluanga, J.; Aswin, S. K.; Rudrapal, M.; Chetia, D. Antimalarial Flavonoid-Glycoside from Acacia Pennata with Inhibitory Potential Against PfDHFR-TS: An In-Silico Study. Biointerface Res. Appl. Chem. 2021, 12, 4871–4887.

 ETFLIN
Notification
ETFLIN
Notification