Ethyl Acetate Fraction of Gynura procumbens Mitigates Hyperglycemia, Dyslipidemia, and Tissue Damage in Streptozotocin-Induced Diabetic Rats
by Yani Mulyani ★ , Marita Kaniawati, Widhya Aligita, Eka Rahmat Nugraha
Academic editor: Mohd Shahezwan Abd Wahab
Sciences of Pharmacy 4(4): 231-238 (2025); https://doi.org/10.58920/sciphar0404371
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
31 May 2025
21 Aug 2025
16 Sep 2025
07 Oct 2025
Abstract: Dyslipidemia is a lipid metabolism disorder frequently associated with diabetes mellitus and known to increase cardiovascular risk. Although Gynura procumbens has been reported to possess antidiabetic and antidyslipidemic properties, studies focusing on the specific effects of its ethyl acetate fraction remain limited. This study investigated the therapeutic potential of the ethyl acetate fraction of ethanol extract from G. procumbens in streptozotocin-induced diabetic rats, particularly its impact on blood glucose, lipid profiles, and histological changes in pancreatic and aortic tissues. Thirty rats were randomly assigned to six groups: normal control, diabetic control, glibenclamide, simvastatin, and extract-treated groups at 100 and 150 mg/kg body weight. After 28 days of treatment, the 150 mg/kg dose significantly reduced blood glucose by 61.3%, total cholesterol by 42.1%, triglycerides by 47.3%, and LDL by 55.0%, while increasing HDL by 6.3% compared to diabetic control (p < 0.05). Histological analysis demonstrated regeneration of pancreatic β-cells and improvement of vascular structure in the aorta. These findings suggest that the ethyl acetate fraction of G. procumbens may serve as a promising natural agent for managing diabetes and dyslipidemia.
Keywords: Flavonoid compoundsInsulin secretionLipid metabolismPancreatic β-cell regenerationOxidative stress reductionVascular protection
Introduction
According to global data, the prevalence of diabetes mellitus patients with dyslipidemia worldwide was approximately 45% in 2020, with a value of 35% being recorded in Indonesia (1). Diabetes mellitus is a metabolic disorder marked by persistently elevated blood glucose levels due to impaired insulin secretion, reduced insulin sensitivity, or a combination of both (2). Similarly, dyslipidemia is defined as a disorder of lipid metabolism, characterized by elevated levels of total cholesterol, low-density lipoprotein (LDL), and triglycerides, along with reduced high-density lipoprotein (HDL) levels (3) The coexistence of these conditions increases the risk of macrovascular and microvascular complications in diabetic patients, including atherosclerosis, cardiovascular disease, nephropathy, and retinopathy (4)
In a previous study by Mulyani et al. (2023), G. procumbens (Lour.) Merr has shown effectiveness in lowering fasting blood glucose, total cholesterol, triglyceride, and LDL levels, while enhancing HDL concentrations in diabetic mouse models. Traditionally, this plant is also used to reduce body heat and treat various ailments such as diabetes, spleen disorders, kidney and heart diseases (5). Pharmacologically, it has demonstrated multiple pharmacological activities, including blood glucose-lowering, antihyperlipidemic, hepatoprotective, antimicrobial, and antihypertensive effects (6).
Phytochemical investigations conducted by Jobaer et al. (2023) on the methanolic extract of G. procumbens leaves identified several key bioactive compounds, including lupeol, β-amyrin, stigmasterol, phytol, friedelanol acetate, and a mixture of stigmasterol and β-sitosterol (7). Notably, phytol and friedelanol acetate were reported in this species for the first time. These compounds belong to classes of triterpenoids and sterols, which are known to contribute to antioxidants, antihyperlipidemic, and antidiabetic properties through modulation of insulin signaling and lipid metabolism. Numerous studies have indicated that streptozotocin (STZ), a compound derived from Streptomyces achromogenes, possesses broad-spectrum antibiotic activity and is commonly employed to induce experimental models of diabetes mellitus due to its selective cytotoxicity toward pancreatic β-cells (8).
Previous studies have demonstrated that G. procumbens effectively reduced fasting blood glucose, total cholesterol, triglycerides, and LDL levels while elevating HDL concentrations in streptozotocin- and alloxan-induced diabetic models, thereby confirming its potential as an antidiabetic and antidyslipidemic agent (9, 10).
Despite these promising findings, most studies to date have utilized crude ethanol extracts, with limited exploration of specific solvent fractions that may yield higher concentrations of active constituents. Among these, the ethyl acetate fraction is of particular interest due to its ability to isolate semi-polar compounds, including flavonoids with known antidiabetic and antidyslipidemic properties (11, 12). Furthermore, there is a scarcity of in vivo studies examining the histological effects of this fraction on pancreatic and aortic tissues, which are critical in understanding tissue-level protection and regeneration.
This study investigates the antidiabetic and antihyperlipidemic potential of the ethyl acetate fraction of G. procumbens ethanol extract in a streptozotocin-induced diabetic rat model. The novelty of this study lies in its focus on a specific bioactive fraction, dose-response analysis, and the simultaneous assessment of biochemical markers and histopathological changes, an approach not widely reported in existing literature.
Materials and Methods
Materials
The materials used included G. procumbens leaves, approximately 2–3 months old, which were collected from a certified herbal garden in Subang, West Java, Indonesia, and authenticated at the Herbarium Bandungense, Universitas Padjadjaran. Ethanol 96% (Bratachem, Indonesia), ethyl acetate (Merck, Germany), and n-hexane (Merck, Germany) were used as solvents. Glibenclamide® (Actavis, Indonesia) and simvastatin® (Hexpharm Jaya, Indonesia) were used as standard drugs. Sodium carboxymethyl cellulose (Na-CMC) (Bratachem, Indonesia) was used as a suspending agent. Streptozotocin (Sigma-Aldrich, USA) was used for diabetes induction. Distilled water was obtained from the Pharmaceutical Laboratory of Universitas Bhakti Kencana. Total cholesterol and triglyceride reagents were from Proline® (Biotechnologies, Indonesia), and HDL cholesterol reagent was from Sekisui® (Sekisui Diagnostics, Japan).
Extraction and Fractionation
Fresh leaves of G. procumbens were washed, air-dried at room temperature (25–28 °C) for 7 days, and then oven-dried at 40 °C until a constant weight was obtained. The dried leaves were powdered using a mechanical grinder and passed through a 60-mesh sieve. The powdered simplicia was macerated with 96% ethanol (1:10 w/v) for 72 h at room temperature with occasional stirring, followed by filtration. The filtrate was concentrated under reduced pressure using a rotary evaporator at 40°C, 90 rpm, with a pressure of approximately 80 mbar. The residue was then fractionated successively with n-hexane and ethyl acetate to obtain the ethyl acetate fraction.
Antidiabetic and Antidyslipidemic Activity
Experimental Animals
A total of 30 male Wistar rats (Rattus norvegicus), weighing approximately 200 g and aged 2–3 months, were used in this experiment. The animals were obtained from the Faculty of Pharmacy, Bhakti Kencana University, Indonesia. Five animals were assigned to each group, selected to ensure comparable age and body weight. Male rats were chosen because of their more stable metabolic profiles compared to females. All animal handling and preparation procedures adhered to the ARRIVE guidelines and institutional ethical standards and were approved by the Study Ethics Commission of Padjadjaran University (Registration Number 2401080024).
Animals were allowed to adapt for seven days under standardized laboratory conditions (temperature 22 ± 2 °C, 12-hour photoperiod, and 55–65% humidity). To ensure reliable biochemical results, rats were fasted for 16 h before the test while water remained available.
Diabetes Mellitus Induction
Experimental diabetes was induced by administering streptozotocin intraperitoneally at a dose of 35 mg/kg body weight. The compound was prepared by dissolving the streptozotocin powder in a 0.1 M citrate buffer solution at pH 4.5. All rat groups, except for the normal control group, were treated with this dose to establish a diabetic model (8, 13). Seventy-two h post-induction, blood samples were collected from the tail veins of the rats, and glucose levels were assessed using a glucometer. Rats exhibiting blood glucose concentrations exceeding 200 mg/dL were classified as diabetic and included in the treatment phase. These animals subsequently received the test formulation orally for a duration of 28 days (14).
After confirming diabetes induction (blood glucose levels >200 mg/dL), the animals were randomly assigned to six groups (n = 5 per group): (1) normal control, (2) diabetic control, (3) glibenclamide-treated (0.65 mg/20 g BW), (4) simvastatin-treated (0.52 mg/20 g BW), (5) FEADSN 100 mg/kg BW, and (6) FEADSN 150 mg/kg BW. The two FEADSN dose levels (100 and 150 mg/kg BW) were selected based on preliminary dose-optimization studies conducted in our laboratory. All treatments were administered orally once daily for 28 consecutive days (15).
Blood Sugar Level Test
Following induction, rats received oral administration of the ethyl acetate fraction derived from the ethanol extract of G. procumbens leaves. Blood glucose measurements were conducted at four intervals: before induction (T0), three days post-induction (T3), and on days 14 (T14) and 28 (T28) after treatment initiation. Glucose levels were determined using a digital glucometer (GlucoDr®, Allmedicus, South Korea) by collecting a drop of blood from the tail vein and applying it to a test strip. The readings, displayed in mg/dL, were recorded manually (16).
Lipid Profile Test
Antidyslipidemia testing was performed on day 3 after induction (T0) and day 28 after treatment (T28). Blood was drawn from the orbital sinus using a microcapillary tube and was allowed to clot for 10 min. The clotted samples were subsequently centrifuged at 5000 rpm for 5 min to separate the serum. Total cholesterol levels were measured using the Cholesterol Oxidase–Peroxidase 4-Aminoantipyrine Phenol (CHOD-PAP) enzymatic method (Diasys®, Germany), while triglyceride (TG) concentrations were determined through the Glycerol-3-Phosphate Oxidase–Peroxidase (GPO-PAP) method. High-density lipoprotein cholesterol (HDL-C) was assessed using an HDL-specific reagent kit (Sekisui Diagnostics®, Japan), and low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald formula. Quality control and calibration procedures were performed using 3 µL of control and calibrator solutions (Proline®, Indonesia), following the manufacturer’s instructions.
Histopathology of Pancreas and Aorta
After the treatment period, the pancreas and aorta of rats were taken for histological analysis by euthanizing the rats with CO2 gas to make them unconscious. Subsequently, an incision was made in the abdomen to the chest. The pancreas and aorta were removed for examination. The histopathology process was carried out with paraffin, starting with washing the aorta with 0.9% Physiological NaCl, 10% formalin fixation for 48 h, dehydration with alcohol, and thin sectioning with a rotary microtome, followed by Hematoxylin and Eosin staining for the aorta and Gomori for pancreas (5, 17). Examination of the aorta includes wall thickness, lumen diameter, and aortic endothelial cell damage. Assessments are carried out to obtain accurate data.
Data Analysis
The blood glucose and lipid profile data were subjected to one-way Analysis of Variance (ANOVA), followed by Least Significant Difference (LSD) post hoc testing to identify statistically significant differences among the treatment groups. All values were reported as mean ± standard deviation (SD), and statistical significance was defined as a p-value less than 0.05.
Results and Discussions
Glucose Levels
Glucose levels were presented in Table 1, showing that the streptozotocin-induced group on the 3rd day experienced an increase in blood glucose levels >200 mg/dL compared to the normal group and was statistically different (p < 0.05). Normal blood sugar levels in rats ranged from 50 to 195 mg/kgBW. Subsequently, there was an increase in blood glucose levels through its working mechanism of forming free radicals. This then caused damage to pancreatic β cells and disrupted insulin production (18). In approximately 2 to 4 days, there was damage to pancreatic β-cells after administration of streptozotocin, characterized by swelling of the pancreas and degeneration of β-cells of the islets of Langerhans (19). A reduction in fasting blood glucose levels was observed on days 14 (T14) and 28 (T28) in the groups treated with the ethyl acetate fraction of G. procumbens leaves and the glibenclamide comparator, with the most notable effect seen at the dose of 150 mg/kg body weight. This finding demonstrated a statistically significant decline in blood glucose when compared to the diabetic control group (p < 0.05). Furthermore, the results revealed that the ethyl acetate fraction of leaves had the potential as an antidiabetic agent from flavonoid compounds to increase insulin secretion and glucose uptake by tissues, specifically muscle and fat. A reduction in blood glucose levels was achieved through the stimulation of pancreatic β-cells. This effect was mediated by an enhancement in insulin sensitivity, which allowed cells to utilize glucose more efficiently as an energy source in rats induced with streptozotocin (20). In the glibenclamide-treated group, the reduction in blood glucose was attributed to its mechanism of action, in which insulin secretion was stimulated through the closure of ATP-sensitive potassium channels in pancreatic β-cells (21). In the glibenclamide-treated group, the reduction in blood glucose was attributed to its mechanism of action, in which insulin secretion was stimulated through the closure of ATP-sensitive potassium channels in pancreatic β-cells (21). These findings indicate that both glibenclamide and the ethyl acetate fraction of G. procumbens can significantly improve glucose control in streptozotocin-induced diabetic rats(22-24), yet limited data exist on the activity of its ethyl acetate fraction.
The hypoglycemic activity observed here may be attributed to flavonoid constituents such as lupeol and β-sitosterol, which have been reported to improve insulin secretion and glucose uptake by peripheral tissues (7). Our findings are in agreement with Tan H et al. (2016), who highlighted multiple pharmacological activities of G. procumbens, including antidiabetic potential (6).
Total Cholesterol Levels
Total cholesterol level measurements compared to the normal and streptozotocin-induced groups were more stable and had lower values (Table 2). Following streptozotocin administration, a significant elevation in total cholesterol levels was observed in the diabetic group when compared to the normal control (p < 0.05). A notable reduction in total cholesterol concentrations was found in the groups treated with simvastatin and the ethyl acetate fraction of G. procumbens ethanol extract, showing a statistically significant difference relative to the diabetic group (p < 0.05). These findings indicated the potential antidyslipidemic activity of the ethyl acetate fraction, likely attributed to its flavonoid constituents, which have been reported to inhibit the activity of HMG-CoA reductase, a key enzyme involved in hepatic cholesterol biosynthesis (25). Furthermore, this was a major enzyme in cholesterol synthesis in the liver. By inhibiting the activity of this enzyme, the production of new cholesterol could be reduced; therefore, it can reduce total cholesterol levels in the blood. The average total cholesterol levels in the streptozotocin-induced group indicated a significant increase. Meanwhile, the group treated with simvastatin and the ethyl acetate fraction of ethanol extract of G. procumbens leaves at doses of 100 and 150 mg/dL showed a decrease in total cholesterol levels. These results revealed that this fraction at a dose of 150 mg/dL could contribute well to reducing total cholesterol levels in streptozotocin-induced rats.
Blood Glucose Level (mg/dL) (Mean ± SD) | % Increase | ||||
T0 (mg/dL) | T3 (mg/dL) | T14 (mg/dL) | T28 (mg/dL) | ||
Normal | 109.25 ± 9.77 | 108.50 ± 10.84a | 109.50 ± 9.43a | 110.75 ± 10.56a | 1.35 |
Diabetic control | 103.00 ± 12.72 | 272.25 ± 9.53b | 295.50 ± 3.10b | 325.50 ± 16.34b | 79.25 |
Glibenclamide | 116.25 ± 7.41 | 270.25 ± 5.79b | 154.00 ± 10.89ab | 111.75 ± 11.32a | -56.31 |
FEADSN 100 mg/KgBW | 101.25 ± 5.56 | 271.50 ± 10.66b | 179.00 ± 8.90ab | 129.25 ± 8.30a | -34.09 |
FEADSN 150 mg/KgBW | 106.50 ± 10.08 | 305.0 ± 11.97ab | 168.00 ± 5.71ab | 125.25 ± 4.11a | -47.99 |
Note: (a) Significantly different from the diabetic control group (p < 0.05); (b) significantly different from the normal group (p < 0.05). T0 represents the initial blood glucose level before streptozotocin (STZ) induction at 30 mg/kg BW; T3 represents the blood glucose level 3 days after STZ induction; T14 represents the blood glucose level 14 days after administration of the test compound; and T28 represents the blood glucose level 28 days after administration of the test compound. FEADSN 1 refers to the ethyl acetate fraction of the ethanol extract of G. procumbens leaves at a dose of 100 mg/kg BW, while FEADSN 2 refers to the same extract at a dose of 150 mg/kg BW. The percentage change was calculated as ((T28 − T3) / T3) × 100, where positive values indicate an increase and negative values indicate a decrease. | |||||
Group | Average total cholesterol levels | ||
T3 (before treatment) | T28 (after treatment) | % Decrease | |
Normal | 32.25 ± 4.17a | 31.75 ± 4.70a | - 2.30 |
Diabetic control | 98.18 ± 11.57b | 108.23 ± 27.15b | 10.23 |
Simvastatin | 89.10 ± 7.42b | 41.18 ± 6.66a | -53.78 |
FEADSN 100 mg/KgBW | 77.75 ± 9.71b | 48.48 ± 5.01a | -37.65 |
FEADSN 150 mg/KgBW | 79.07 ± 7.56b | 45.77 ± 6.80a | -42.11 |
Note: (a) Significantly different from the diabetic control group (p < 0.05); (b) significantly different from the normal group (p < 0.05). T3 represents the total cholesterol level before treatment, and T28 represents the total cholesterol level after 28 days of treatment. FEADSN 1 refers to the ethyl acetate fraction of the ethanol extract of G. procumbens leaves at a dose of 100 mg/kg BW, while FEADSN 2 refers to the same extract at a dose of 150 mg/kg BW. The percentage decrease was calculated as ((T28 − T3) / T3) × 100, where negative values indicate a reduction in cholesterol levels compared with baseline. | |||
Triglyceride Levels
The results of measuring triglyceride levels looked at the normal group, the group that had streptozotocin-induced, and the group treated with ethyl acetate from the ethanol extract of G. procumbens leaves. This is shown in Table 3.
High triglyceride levels occurred because there wasn’t enough insulin. This caused more lipolysis through a hormone-sensitive lipase in fat cells (26). When more fatty acids are released from fat cells, it could boost the production of VLDL in the liver, which has a lot of triglycerides, raising their levels in the blood. Also, people with diabetes had lower levels of lipoprotein lipase, which made lipid metabolism go down (27). No statistically significant variation was found between the effects of simvastatin and the ethyl acetate fraction of G. procumbens, as demonstrated by the analysis (p < 0.05). This indicated that a dose of 150 mg of G. procumbens showed a triglyceride-lowering effect almost equivalent to simvastatin. Flavonoid compounds function as HMG-CoA reductase enzymes in the liver, small intestinal mucosa, and blood. Simvastatin suppressed liver triglyceride synthesis, which caused decreased blood triglyceride levels. Meanwhile, the disease group experienced increased triglyceride levels due to decreased HDL levels.
These findings are consistent with previous studies reporting the antihyperlipidemic potential of G. procumbens. Arifah et al. (2022) reviewed medicinal plants used for diabetes management in Indonesia and highlighted that G. procumbens significantly reduced triglyceride and total cholesterol levels in experimental models (22). Similarly, Algariri et al. (2013) demonstrated that ethanolic extracts of G. procumbens lowered triglycerides and improved lipid profiles in diabetic rats (24). The mechanism is thought to involve flavonoids that inhibit hormone-sensitive lipase and HMG-CoA reductase, thereby reducing VLDL production and enhancing lipid metabolism.
HDL Levels
In type 2 diabetes, dyslipidemia was defined by elevated triglyceride levels, accompanied by decreased HDL and elevated LDL cholesterol levels (28). In streptozotocin-induced rats, lipoproteins contained high concentrations of triglycerides. This caused increased transfer of cholesterol esters from HDL particles to lipoproteins, facilitated by CETP (Cholesteryl Ester Transfer Protein) (28). HDL level measurements in this study were evaluated by comparing the normal control group, the streptozotocin-induced diabetic group, the simvastatin-treated group, and the groups treated with the ethyl acetate fraction of the ethanol extract of G. procumbens leaves (Table 4). An elevation in HDL levels was detected at T3 and T28 in rats treated with simvastatin, indicating the activation of reverse cholesterol transport, a process by which HDL facilitates the movement of cholesterol from peripheral sites to the liver for metabolism. This increase was related to the elevated synthesis of apolipoprotein A-I (Apo-AI), the main component of HDL, which helped form and maintain HDL particles. Meanwhile, the fraction increased HDL function by increasing antioxidant and anti-inflammatory effects, reducing HDL damage, and increasing cholesterol efflux from macrophages to HDL (29).
Group | Average Triglyceride Levels | ||
T3 (before treatment) | T28 (after treatment) | % Decrease | |
Normal | 33.80 ± 8.16a | 39.40 ± 3.34a | 16.56 |
Diabetic control | 106.85 ± 19.97b | 105.05 ± 12.28b | -1.68 |
Simvastatin | 95.43 ± 18.72b | 42.90 ± 14.28a | -55.04 |
FEADSN 100 mg/KgBW | 99.97 ± 17.72b | 52.65 ± 6.41a | -47.33 |
FEADSN 150 mg/KgBW | 92.92 ± 19.44b | 47.075 ± 6.10a | - 49.34 |
Note: (a) Indicates a significant difference from the diabetic control group (p < 0.05); (b) indicates a significant difference from the normal group (p < 0.05). T3 represents the cholesterol level before treatment, and T28 represents the cholesterol level after treatment. FEADSN refers to the ethyl acetate fraction of the ethanol extract of G. procumbens leaves. The percentage change was calculated as ((T28 − T3) / T3) × 100, where positive values indicate an increase and negative values indicate a decrease. | |||
Group | Average HDL levels | ||
T3 (before treatment) | T28 (after treatment) | % increase | |
Normal | 20.73 ± 3.80a | 18.90 ± 3.67 | -9.66 |
Diabetic control | 17.40 ± 3.80b | 14.95 ± 1.06 | -16.39 |
Simvastatin | 13.33 ± 2.69b | 15.45 ± 6.27 | 13.75 |
FEADSN 100 mg/KgBW | 15.45 ± 3.71b | 17.15 ± 2.61 | 9.91 |
FEADSN 150 mg/KgBW | 14.80 ± 1.98b | 15.80 ± 3.74 | 6.33 |
Note: (a) Indicates a significant difference from the diabetic control group (p < 0.05); (b) indicates a significant difference from the normal group (p < 0.05). T3 represents the HDL level before treatment, and T28 represents the HDL level after treatment. FEADSN refers to the ethyl acetate fraction of the ethanol extract of G. procumbens leaves. The percentage change was calculated as ((T28 − T3) / T3) × 100, where positive values indicate an increase and negative values indicate a decrease. | |||
Interestingly, the FEADSN 100 mg/kg group showed a higher elevation in HDL levels compared to the FEADSN 150 mg/kg group. This paradoxical finding may be explained by a non-linear, dose-dependent pharmacological response, which has been observed in several plant-derived flavonoids. At moderate doses, flavonoids can enhance reverse cholesterol transport and upregulate apolipoprotein A-I expression, leading to increased HDL concentrations. However, at higher doses, excessive antioxidant or metabolic modulation may trigger compensatory mechanisms that attenuate HDL elevation, a phenomenon often referred to as a “bell-shaped” dose-response curve.
Similar observations have been reported in a previous study noted that dietary flavonoids improve HDL metabolism and function, but highlighted that the response may plateau or diminish at higher doses (12). Al-ishaq et al. (2019) described that flavonoids exert antidiabetic and lipid-modulating effects through insulin signaling and lipid metabolism, but excessive concentrations could paradoxically impair lipid regulation (11). These findings suggest that the optimal activity of G. procumbens extract may occur at moderate doses rather than at higher concentrations.
Unexpectedly, the normal control group also exhibited a slight decrease in HDL levels from T3 to T28. This reduction may be attributed to physiological variations in lipid metabolism that naturally occur over time, even in healthy animals. Previous studies have reported that HDL concentrations in rodents are influenced by dietary composition, age, and stress during handling and fasting procedures (28). Moreover, Wang et al. (2012) demonstrated that streptozotocin-induced diabetic rats, as well as normal rats, may present fluctuations in tissue cholesterol and HDL levels due to metabolic adaptations (29). These findings suggest that the observed decline in HDL among the normal group may reflect natural metabolic adjustments rather than pathological changes.
LDL Levels
The results, as shown in Table 5, revealed increased levels in the group given streptozotocin induction due to the high levels of triglycerides and cholesterol in the liver in VLDL, IDL, and LDL (30). This indicated that the ethyl acetate fraction of ethanol extract of G. procumbens leaves could potentially reduce LDL levels in streptozotocin-induced rats, with a higher dose (150 mg) showing better effectiveness than a lower dose (100 mg). The increased LDL levels in the disease group showed a lipid metabolism disorder due to diabetes. This indicated that streptozotocin induction could significantly increase LDL levels.
A significant elevation in LDL concentrations was recorded following 28 days of intervention in the disease group (P < 0.05), particularly among the rats receiving simvastatin and those administered the ethyl acetate fraction of G. procumbens ethanol extract at both 100 and 150 mg/kg BW. Furthermore, there was a decrease in the test group simvastatin, which functioned as a competitive inhibitor of HMG-CoA reductase enzyme, accelerating cholesterol production. Simvastatin reduced cholesterol synthesis in the liver by blocking this enzyme, thereby reducing LDL cholesterol levels in the bloodstream (31). FEADSN was rich in flavonoid compounds that could reduce LDL levels. Flavonoids operate by inhibiting LDL oxidation, increasing lipid metabolism, and reducing inflammation in the body (12).
Group | Average LDL levels | ||
T3 (before treatment) | T28 (after treatment) | % decrease | |
Normal | 14.98 ± 7.42a | 14.85 ± 4.11a | 0.90 |
Diabetic control | 59.41 ± 10.87b | 70.23 ± 28.62b | -18.21 |
Simvastatin | 56.69 ± 7.85b | 17.15 ± 11.21a | 69.76 |
FEADSN 100 mg/KgBW | 42.31 ± 14.29b | 20.80 ± 4.66a | 50.85 |
FEADSN 150 mg/KgBW | 45.69 ± 9.10b | 20.56± 7.32a | 55 |
Note: (a) Indicates a significant difference from the diabetic control group (p < 0.05); (b) indicates a significant difference from the normal group (p < 0.05). T0 represents the initial cholesterol level after streptozotocin (STZ) induction at 35 mg/kg BW; T3 represents the cholesterol level before treatment; and T28 represents the cholesterol level after treatment. FEADSN refers to the ethyl acetate fraction of the ethanol extract of G. procumbens leaves. The percentage decrease in LDL was calculated as ((T3 − T28) / T3) × 100, expressed as positive values, where higher percentages indicate greater reductions in LDL levels. | |||
Group | The Islets Area of Langerhans (mm)2 | Total | |
α Cell | β Cell | ||
Normal | 5.31 ± 1.60a | 47.00 ± 12.49a | 130.20 ± 53.15a |
Diabetic control | 2.33 ± 1.04b | 17.80 ± 6.30b | 49.40 ± 15.17b |
Glibenclamide | 4.44 ± 1.31a | 14.0 ± 6.96b | 103.60 ± 25.61a |
FEADSN 100 mg/KgBW | 2.64 ± 0.60 | 17.40 ± 16.06b | 99.20 ± 14.44a |
FEADSN 150 mg/KgBW | 3.13 ± 1.01 | 20.00 ± 9.40b | 112.20 ± 12.79a |
Note: (a) Indicates a significant difference from the diabetic control group (p < 0.05); (b) indicates a significant difference from the normal group (p < 0.05). The islet area includes measurements of α-cells, β-cells, and total pancreatic islet area. FEADSN refers to the ethyl acetate fraction of the ethanol extract of G. procumbens leaves at doses of 100 mg/kg BW and 150 mg/kg BW, respectively. | |||
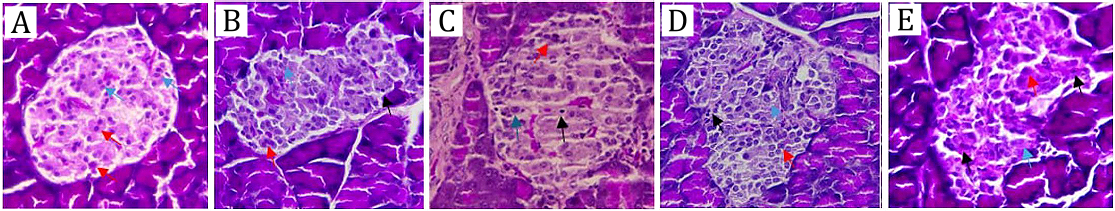
Histopathology of Pancreas
Histopathological examination was conducted on pancreatic tissues obtained from streptozotocin-induced experimental rats to assess the effects of administering the ethyl acetate fraction of G. procumbens ethanol extract. The evaluation focused on determining improvements in pancreatic structure by observing the cellular density within the islets of Langerhans. From the results shown in Table 6, A marked reduction in the islets of Langerhans area, as well as in the number of α and β cells, was observed in the streptozotocin-induced group when compared to the normal control (32). However, improvement was noted in the group treated with the ethyl acetate fraction of G. procumbens, particularly at the dose of 150 mg/kg BW, where the number of β cells approached values similar to those observed in the normal group (Figure 1). These results revealed that administering the fraction could improve pancreatic structure and increase the number of β cells, which played an important role in insulin production.
Similar protective and regenerative effects of G. procumbens on β-cells have been reported in earlier studies. Algariri et al. (2013) observed that ethanolic extracts of G. procumbens improved pancreatic histoarchitecture in diabetic rats (24). Jobaer et al. (2023) further identified bioactive compounds such as lupeol and β-sitosterol that may contribute to β-cell regeneration via antioxidant and anti-apoptotic mechanisms (7). Comparable findings were also highlighted by Tan et al. (2016), who emphasized the plant’s potential in preserving pancreatic function in metabolic disorders (6).
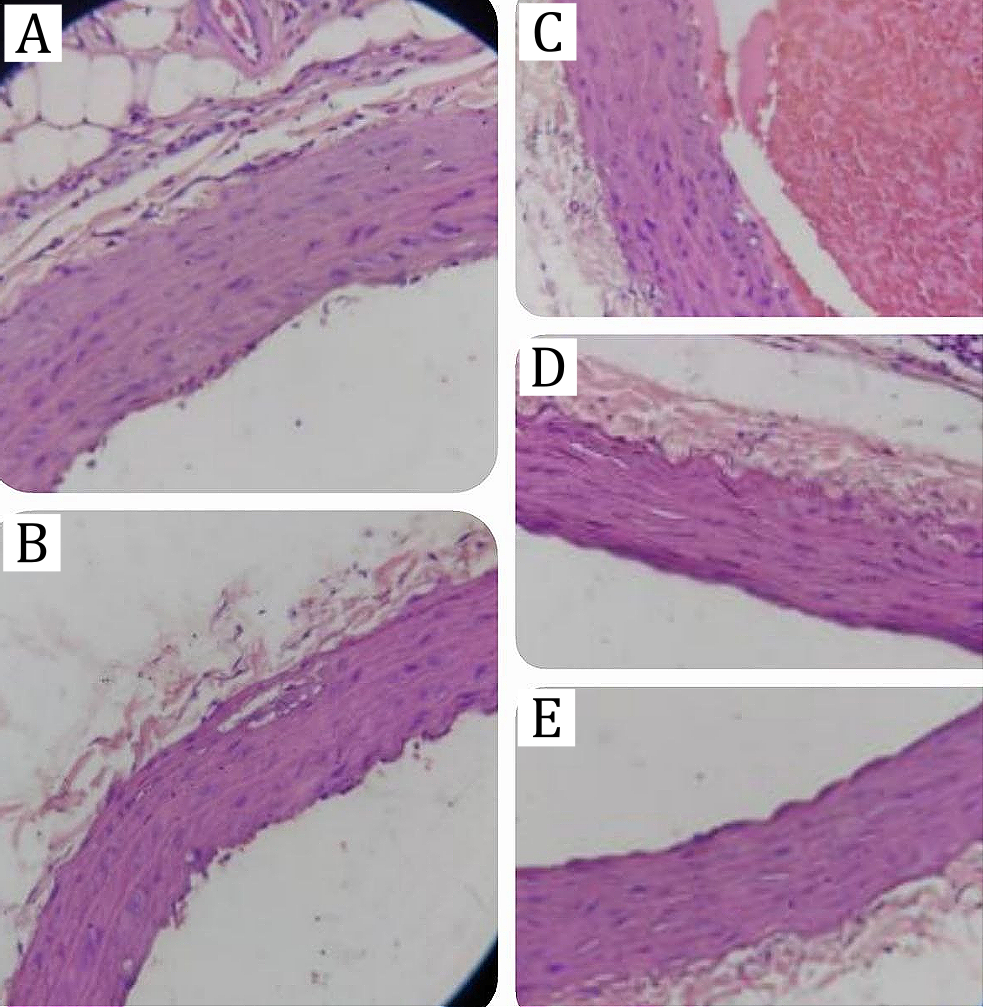
Histopathology of the Aorta
The histopathological analysis of the aorta (Figure 2) revealed that the normal control group exhibited intact endothelial and smooth muscle layers, while the diabetic control group showed marked structural disruption, including irregular arrangement of vascular smooth muscle cells, endothelial damage, and prominent vacuolization. These pathological changes are consistent with vascular injury commonly observed in diabetic conditions, which is often associated with increased LDL oxidation and subsequent foam cell formation (33).
Treatment with simvastatin and the ethyl acetate fraction of G. procumbens improved the vascular architecture, with the 150 mg/kg BW dose showing the most notable effect. Minimal vacuolization and restoration of smooth muscle integrity were observed, suggesting that the fraction protects against oxidative stress and lipid peroxidation-induced vascular injury. These findings are in line with previous reports demonstrating that G. procumbens exerts vasculoprotective and antihyperlipidemic effects through flavonoid-mediated antioxidant and anti-inflammatory mechanisms (6, 7)
Comparable evidence from streptozotocin-induced diabetic models showed that hypertriglyceridemia promotes endothelial dysfunction and accelerates atherogenesis viafoam cell accumulation (29, 30). By reducing triglyceride and LDL levels, the ethyl acetate fraction of G. procumbens may limit LDL oxidation and subsequent macrophage uptake, thereby preventing vascular degeneration. Collectively, these results support the hypothesis that G. procumbens at an optimal dose not only modulates lipid metabolism but also confers direct histological protection to vascular tissues.
Conclusion
In conclusion, the ethyl acetate fraction of G. procumbens leaves demonstrated significant antidiabetic and antidyslipidemic effects in streptozotocin-induced diabetic rats. Treatment, particularly at the 150 mg/kg body weight dose, effectively reduced blood glucose, total cholesterol, triglyceride, and LDL levels, while enhancing HDL concentrations. Moreover, this fraction contributed to histological improvement by reducing vacuolization in the aortic tunica media and promoting pancreatic islet recovery, notably through regeneration of insulin-secreting β-cells. These findings highlight the potential of the ethyl acetate fraction of G. procumbens as a promising natural therapeutic candidate for the management of diabetes and its associated dyslipidemia.
Abbreviations
FEADSN = Ethyl Acetate Fraction of Ethanol Extract of G. procumbens, STZ = Streptozotocin, LDL = Low-Density Lipoprotein, HDL = High-Density Lipoprotein.
Declarations
Ethics Statement
All experimental procedures involving animals were conducted in accordance with the ethical standards for the care and use of laboratory animals. The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Padjadjaran University with Registration Number 2401080024. Efforts were made to minimize animal suffering, and the number of animals used was kept to the minimum necessary to achieve statistical significance.
Data Availability
All relevant data supporting the findings of this study are included within the manuscript.
Funding Information
Research activities was funded by the Directorate of Research and Community Service, Bhakti Kencana University (Grant No. 012/PEN.DRPM/UBK/VII/2024).
Conflict of Interest
The authors declare no conflicting interest.
References
- IDF. IDF Diabetes Atlas 2021 [Internet]. 2021. Available from: https://diabetesatlas.org/atlas/tenth-edition/
- Association AD. Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):1–232.
- Gopan Y, Kamath TS. Ayurvedic management of dyslipidemia – a conceptual analysis. Int Ayurvedic Med J. 2024;12(1):237–242.
- Usman MS, Khan MS, Butler J. The interplay between diabetes, cardiovascular disease, and kidney disease. ADA Clin Compend. 2021;2021(1):13–18.
- Mulyani Y, Hasimun P, Fazila E, Asfa K. Antidiabetic and antidyslipidemic activities of combined Bandotan (Ageratum conyzoides) and Sambung Nyawa (Gynura procumbens) ethanol extracts in insulin-resistant rat models. World J Pharm Res. 2023;12:1089.
- Tan HL, Chan KG, Pusparajah P, Lee LH, Goh BH. Gynura procumbens: an overview of the biological activities. Front Pharmacol. 2016;7:52.
- Jobaer MA, Ashrafi S, Ahsan M, Hasan CM, Rashid MA, Islam SN, et al. Phytochemical and biological investigation of an indigenous plant of Bangladesh, Gynura procumbens (Lour.) Merr.: drug discovery from nature. Molecules. 2023;28(10):4186.
- Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc. 2021;1(4).
- Mulyani Y, Mastura N, Rosilopya S, Suhardiman A, Fajarwati K, Sutrisno E. Combined antidiabetic and antidyslipidemic activity of Ageratum conyzoides and Gynura procumbens in alloxan-induced diabetic rats. Maj Obat Tradis. 2023;28(2):112–121.
- Algariri K, Meng KY, Atangwho IJ, Asmawi MZ, Sadikun A, Murugaiyah V, et al. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pac J Trop Biomed. 2013;3(5):358–366.
- Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K, Büsselberg D. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9(9):430.
- Millar CL, Duclos Q, Blesso CN. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr. 2017;8:226–239.
- Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Allah Verdi A, et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22(2):60–64.
- Ogar I, Egbung GE, Nna VU, Iwara IA, Itam E. Anti-hyperglycemic potential of Hyptis verticillata Jacq in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018;107:1268–1276.
- Conour LA, Murray KA, Brown MJ. Preparation of animals for research—issues to consider for rodents and rabbits. ILAR J. 2006;47:283–293.
- Bhowmik A, Kabir Y, Rokeya B. Glycemic, insulinemic, lipidemic and antioxidant status of nSTZ rats after chronic administration of Cicer arietinum extract. Metabolomics (Los Angel). 2016;6:3.
- Costa CJS, Wadt D, Conti LC, de Albuquerque Landi MF, Cintra L, de Oliveira FA, et al. Histological alterations in the internal organs of Wistar Han rats (Rattus norvegicus) euthanized by five different methods. J Am Assoc Lab Anim Sci. 2024;63(1):81.
- Li Y, Zhang Y. The role of Gynura procumbens in the management of diabetes and hyperlipidemia: a review. J Ethnopharmacol. 2020;245:112158.
- Zulkarnain Z. Perubahan kadar glukosa darah puasa pada tikus Sprague Dawley yang diinduksi streptozotocin dosis rendah. J Kedokteran Syiah Kuala. 2013;13(2):77–87.
- Ramadhani MA, Kumalahati A, Jusman AH, L NF. Perbandingan aktivitas penurunan glukosa pada ekstrak dan nanoekstrak daun insulin (Tithonia diversifolia) dengan metode in vitro. Generics J Res Pharm. 2021;1(2):28–36.
- Patanè G, Piro S, Anello M, Rabuazzo AM, Vigneri R, Purrello F. Exposure to glibenclamide increases rat beta cells sensitivity to glucose. Br J Pharmacol. 2000;129(5):887.
- Arifah FH, Nugroho AE, Rohman A, Sujarwo W. A review of medicinal plants for the treatment of diabetes mellitus: the case of Indonesia. S Afr J Bot. 2022;149:537–558.
- Saputra IPBA, Arjita IPD. The potential of flavonoid derivative compounds as inhibitors of the HMG-CoA reductase enzyme for candidate of hypercholesterolemia drugs. J Penelit Pendidik IPA. 2024;10(5):2286–2293.
- Althaher AR. An overview of hormone-sensitive lipase (HSL). Sci World J. 2022;2022:1964684.
- Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14(1):121.
- Schofield JD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes dyslipidemia. Diabetes Ther. 2016;7(2):203.
- Wang XT, Li J, Liu L, Hu N, Jin S, Liu C, et al. Tissue cholesterol content alterations in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2012;33(7):909–917.
- Kako Y, Huang LS, Yang J, Katopodis T, Ramakrishnan R, Goldberg IJ. Streptozotocin-induced diabetes in human apolipoprotein B transgenic mice: effects on lipoproteins and atherosclerosis. J Lipid Res. 1999;40(12):2185–2194.
- Adams SP, Alaeiilkhchi N, Wright JM. Simvastatin for lowering lipids. Cochrane Database Syst Rev. 2023;2023(2).
- Nahdi AMTA, John A, Raza H. Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β-cells. Oxid Med Cell Longev. 2017;2017.
- Watanabe T, Fan J. Foam cells: mechanisms of cholesterol uptake, storage, and extracellular release, and their pathophysiological significance. Atherosclerosis. 2025;85–95.

 ETFLIN
Notification
ETFLIN
Notification







