Bambusa vulgaris: A Comprehensive Review of Its Traditional Uses, Phytochemicals and Pharmacological Activities
by Jeba Akhtar, Lima Patowary ★
Academic editor: James H. Zothantluanga
Sciences of Phytochemistry 1(2): 68-75 (2022); https://doi.org/10.58920/sciphy01020011
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
15 Oct 2022
10 Nov 2022
12 Nov 2022
22 Nov 2022
Abstract: A versatile plant with many purposes, Bambusa vulgaris is primarily known for its industrial applications, but it is also becoming acknowledged as a possible source of bioactive substances and as a functional food. Every component of the bamboo plant, including the rhizome, culm shavings, leaves, roots, shoots, and seeds, has potential medical uses. This review aims to provide an insight into the traditional uses, and the various pharmacological activities exhibited by B. vulgaris extracts like analgesic, antihyperglycemic, antipyretic, anti-inflammatory, antioxidant, antimicrobial, antiviral, hepatoprotective, anti-amnesic, etc. It also has immense potential to be used as an important functional food as it has a high content of useful proteins, carbohydrates, high fiber content, and very low fat.
Keywords: Bambusa vulgarisEthnopharmacologyNutritive valueBioactive compounds
Introduction
B. vulgaris is an erect, evergreen, clump-forming bamboo growing 15 - 20 metres tall (Figure 1). It grows in loose clumps that are free of thorns, has dark green leaves and lemon-yellow stems mostly with green stripes. The stems are initially tough, not straight or can split easily, stiff in nature, and have thick walls with narrow lanceolate leaves. The densely tufted stems are 4–10 cm thick and reach heights upto 10–20 m. The trunk can be flexible (alternately bent in various directions) or straight, drooping at the ends. The walls of the trunk are quite thick, and nodes grew marginally. The internodal segment is 20 to 45 cm. There may be sprouting of a few branches between the middle trunk nodes to the top (1). According to CABI (Invasive Species Compendium), new bamboo culms that emerge from the ground (bamboo sprouts) are the edible shoots of several bamboo species particularly B. vulgaris and Phyllostachys edulis.
It's a vegetable that's used in a variety of Asian dishes and broths. They are soldin a variety of processed shapes andcome in fresh, dried, and canned varieties (2). Among all the different species of bamboo shoots available in the world, only some of them are edible. Out of the 136 species of bamboo that can be found in India, B. pallida, B. tulda, B. polymorpha, B. balcooa, Dendrocalamus hamiltonii, Dendrocalamus giganteus, and Melocanna bambusoides are the most popular edible bamboo species (3). The genera of bamboo shoots which are edible available in the USA are Phyllostachys, the important being Phyllostachys dulcis, Phyllostachys edulis, Phyllostachys bambusoides, Phyllostachys pubescens, Phyllostachys nuda and Phyllostachys viridis (4).

Figure 1. (A) Golden bamboo (B. vulgaris) and (B) close-up view of golden bamboo stems.
Scientific Classification
- Kingdom: Plantae
- Clade: Tracheophytes
- Clade: Angiosperms
- Clade: Monocots
- Clade: Commelinids
- Order: Poales
- Family: Poaceae
- Genus: Bambusa
- Species: B. vulgaris
- Binomial name: Bambusa vulgaris.
- Synonym: Bambusa auriculata, Gigantochloa auriculata, B. striata
- Local names: Baah Gaz (Assam), Tama (Nepal), Baseer Korool (Bengali).
Geographical Location
Bamboo grows worldwide in at least 37 million hectares and covers 3.2% of forest areas of their host countries or about 1% of the global forest area. The southern tropical region of Asia contains about 80% of the area covered in bamboo. Bamboo is not very common in Africa or South America. In terms of the diversity of bamboo species, Madagascar has been named the richest nation in Africa. Eleven nations, one from Africa, eight from Asia, and two from Central and South America, contributed information to the Global Forest Resources Assessment 2000 (FRA 2000) on the size of their bamboo forests (5).
In Asia, Africa, and America bamboo is widely distributed. The Southeast Asia monsoon zone (south-eastern China, Indo-China, and the Indian subcontinent) is the world's bamboo distribution center; it is home to 90% of the world's total bamboo forest area as well as 80% of all bamboo species. There are numerous bamboo species in both America and South Africa. It has to do with the history, architecture, andculture of some of these nations. The distribution centre for bamboo is located in Latin America, specifically in the Amazon Basin near the South Tropic of Cancer, which includes Mexico, Guatemala, Costa Rica, Nicaragua, Honduras, Colombia, Venezuela, and Brazil. And the east coast of Madagascar is the centre of African bamboo distribution (6). India is the second richest country in the world, after China, in terms of bamboo genetic resources (5). The bamboo area of the country is estimated to be 15.69 million hectares with a total standing stock of 189 million tons (7).
Traditional Uses
Bamboo is inextricably linked to the cultural, social, and economic conditions of individuals in many Asian countries. It is the fastest-growing, multifunctional woody plant with a plethora of industrial and residential uses. Its use goes beyond just replacing wood in the building, furniture, scaffolding, and flooring; in China and South East Asia, it has traditionally been utilized as a source of food and medicine. The rhizome, culm, bark shavings, shoots, leaves, roots, and seeds of the bamboo plant are all used in medicine (42, 43). Bamboo is currently attracting attention on a global scale for its nutritional and medicinal potential and is crucial to the food, pharmaceutical, and cosmeceutical industries. Bamboo shoots and leaves have excellent medicinal potential and can be used to treat illnesses naturally and sustainably (44, 45).
Bamboo has long been a crucial component of traditional Asian remedies, particularly Chinese and Indian (Ayurvedic) medications (45). Around 10,000 years ago, bamboo's medicinal uses were first documented in India for the preparation of Chyawanprash, a health tonic made from a variety of plants, including bamboo manna to promote youth, beauty, and longevity. Because of its ability to fight stress and slow the signs of aging, Chyawanprash has gained worldwide fame. The traditional Indian medical system of Ayurveda suggests using bamboo and its products, including Banslochan, Tabasheer, and Sitopaladi Churna, to cure a variety of illnesses (44, 45). It is reported that in Pakistan, India, Brazil, and Tanzania it has been traditionally used as an astringent, emmanogogue, and abortifacient (47). Asase et al. (2010) reported that in Ghana it is used as a herbal remedy for the treatment of malaria (48). These modern pharmaceutical preparations made from bamboos, such as bamboo salt, bamboo starch, bamboo extracts, bamboo vinegar, bamboo silica, and more, are made using this ancient knowledge to address a variety of health issues like diabetes, inflammations, constipation, etc. (44, 49, 50).
Phytoconstituents Present in B. vulgaris
Plants are the most abundant source of medications for ancient medical systems, modern medications, nutraceuticals, food supplements, folk remedies, pharmaceutical intermediates, and chemical entities for synthesised drugs (8). The ability to synthesise a wide range of chemical compounds allows plants to protect themselves from predators like insects, fungi, and herbivorous mammals as well as perform vital biological functions. Several active phytocompounds from plants were extracted and characterised to create a number of high-activity profile drugs (9). A variety of plant compounds and extracts have antioxidant or free radical-scavenging capabilities (10). These phytochemicals are separated into primary and secondary metabolites. Both the dry and wet ethanol-extracted leaf samples of B. vulgaris were subjected to a phytochemical analysis to determine their safety for ingestion (11). Table 1 presents the findings of the qualitative examination of B. vulgaris. All of the leaf extract was discovered to contain polyphenol and flavonoids in addition to saponin, general glycoside, coumarin, and cyanogenic glycoside. None of the species contained any remnants of anthraquinone, carotenoid, triterpenoid, steroid, or anthracene glycoside (12). In addition to the leaves and stems, some species of bamboo also have shoots that are valued for their health benefits due to their high protein, carbohydrate, vitamin, fibre, and mineral content and very low-fat content (12).
Table 1. Qualitative analysis of phytochemical constituents of B. vulgaris.
Phytochemical | Detection | Plant Part |
Saponin | + | Leaf |
Tannin | _ | _ |
Terpenes | _ | _ |
Flavonoid | +++ | Leaf |
Phlobatannins | _ | _ |
Alkaloid | _ | _ |
Glycosides | + | Ripe stem |
Resin | + | Culm |
Phenol | + | Leaf |
Steroids | _ | _ |
Proteins | + | Shoot |
Carbohydrates | + | Shoot |
Amino acids | + | Shoot |
Gums & Mucilage | _ | _ |
Non-reducing polysaccharides | _ | _ |
Non-reducing simple sugar | + | Shoot |
Note: + = mildly present; ++ = highly present; +++ = more highly present; - = absent or non-detectable. | ||
Proteins
The protein content of bamboo shoots, which ranges between 1.49 g/100 g to 4.04 g/100 g and 21.1 g/100 g to 25.8 g/100 g on a wet and dry weight basis, is a potential source of proteins for humans. The species and maturity of the bamboo have a significant impact on the amount of protein in a bamboo shoot. The protein level of B. vulgaris was found to be 3.64 g/100 g (14). The composition of proteins in the bamboo shoot is of
Carbohydrates
Bamboo shoots contained polysaccharides, oligosaccharides, and monosaccharides in terms of total carbohydrates. In bamboo shoots, the main polysaccharides are cellulose, hemicellulose, and starch, along with a few other minor complex polysaccharides like glycoproteins. Three oligosaccharides in particular—sucrose, arabinoxylan trisaccharide, tetrasaccharide, and xyloglucan disaccharide—were found to be the main ones in bamboo shoots. Dietary fibre with antioxidants is abundant in bamboo shoots. Bamboo shoots usually contained the monosaccharides fructose and glucose. The carbohydrate content of common species of newly emerged juvenile bamboo shoots usually ranges from 2.0 g/100 g to 9.94 g/100 g (16).
Minerals
According to the results that are currently available, bamboo shoots are a good source of both macro and microelements. The main macro elements are potassium (K), phosphorus (P), sodium (Na), calcium (Ca), and magnesium (Mg), while the main microelements are cobalt (Co), copper (Cu), nickel (Ni), manganese (Mn), selenium (Se), iron (Fe), and zinc (Zn). Most studies found potassium to be the macroelement most abundant in bamboo shoots, followed by phosphorus and magnesium (17).
Vitamins
The majority of studies on vitamins have concentrated on vitamin C (ascorbic acid) and vitamin E. (tocopherol). Vitamins C and E are intimately linked to the body's ability to produce antioxidants in vivo, but vitamin E synergistically with vitamin C strengthen the immune system. Fresh bamboo shoots contain far more vitamin C than vitamin E, which is also true of other common vegetables. Additionally, in some regions, fresh bamboo shoots are a respectable source of β-carotene and B-group vitamins. (18). The amounts of both vitamin C and vitamin E significantly dropped with the age of the shoots, according to a study by Nirmala et al. (2007). Additionally, the amount of vitamin C differed to a variable extent depending on the growth of bamboo shoots' altitude and distinct parts (tip and basal) (51).
Phenolic Compounds
Bamboo shoots contained phenols that were primarily made up of flavonoids and phenolic acids. Bamboo shoots have been found to contain the following phenolic acids: protocatechuic acid, p-hydroxybenzoic acid, catechin, caffeic acid, chlorogenic acid, syringic acid, p-coumaric acid, ferulic acid, gallic acid, and vanillic acid (19). Protocatechuic acid, p-hydroxybenzoic acid, and syringic acid were the three most prevalent substances among them (20). There have been reports of fifteen phenolic acids, including 3-O-caffeolyshikimic acid, chlorogenic acid, p-coumaric acid, 3-p-coumaroylquinic acid, 5-p-coumaroylquinic acid, cryptochlorogenic acid, 1,3- dicaffeoyl quinic acid, 3,5-dicaffeoyl quinic acid, ferulic acid, 3-O-feruloylquinic acid, 5-O-ferul (52).
Flavonoids
Bamboo shoots and leaves contain flavonoids like orientin, isoorientin, isovitexin, vitexin, and tricin (21). Bamboo tissues such as shoots, sheaths, and leaves mostly contained flavonoids in the insoluble form of free aglycone or flavonoid ligands. Apigenin 6,8-di-C-L-arabinopyranoside, 6-C-D-glucopyranosyl-8-C-L-arabinopyranosylchrysin, and kaempferide 3-O-L-rhamnopyranosyl were the seven flavonoids reported (1, 6) 5,7,4′-trihydroxy-3′,5′-dimethoxyflavone, narcissin, rutin, schaftoside, and D-glucopyranoside. The bamboo leaf has a flavone content of 2% to 5%, which has the ability to neutralise active free radicals, prevent sub-nitrification, and lower blood fat (52).
Phytosterols
Plants produce phytosterols in abundance, and B. vulgaris has been shown to be an excellent source of these compounds. To date, bamboo shoots have been found to contain seventeen phytosterols. Six phytosterols, including ergosterol, cholesterol, campesterol, stigmasterol, and β-sitosterol, were typically found in bamboo shoots. The total phytosterol content of bamboo shoots ranged from 66.60 mg/100 g to 242.77 mg/100 g on a dry basis, indicating the plant's ability to provide humans with useful phytosterols. Due to their wide range of health advantages, including their ability to decrease serum cholesterol and their anti-ulcer, anti-cancer, anti-inflammatory, and immunomodulatory properties, phytosterols were regarded as valuable dietary supplements (22).
Glycosides
The presence of cyanogenic glycosides has been reported in B. vulgaris. The cyanogen glycoside taxiphyllin is found in different levels in bamboo shoots (23-26). The β-glycosidase, which is produced in damaged bamboo shoot tissues, reacts with taxiphyllin to form dangerous hydrogen cyanide, whose concentration shouldn't be higher than what is toxic to humans (3). The majority of edible species of bamboo shoots have a significant quantity of cyanogen glycoside, with the shoot tip having the highest concentration. The detailed phytoconstituents that are found in B. vulgaris are provided in Table 2. The chemical structure of different phytocompounds present in B. vulgaris is provided in Figure 2.
Pharmacological Activity
Anti-inflammatory
A study was designed by Carey, W. M. et al. (2009) to investigate the anti-inflammatory effects of B. vulgaris methanol extract (MEBV) in mice. Acute inflammatory models such as formaldehyde-induced paw edema and acetic acid-induced vascular permeability were used to investigate anti-inflammatory effects, as were subacute anti-inflammatory models such as cotton pellet granuloma, plasma MDA estimation, and carrageenan-induced peritonitis (27).
Table 2. Types of phytoconstituents present in B. vulgaris.
Classes | Phytoconstituents |
Carbohydrates | Polysaccharides: cellulose, hemicellulose, Starch & glycoproteins. Oligosaccharides: sucrose, tetrasaccharide, arabinoxylan trisaccharide, xyloglucan disaccharide. Monosaccharides: fructose, glucose |
Amino acids | Arginine (Arg), aspartic acid (Asp), serine (Ser), glycine (Gly), glutamic acid (Glu), alanine (Ala), threonine (Thr), proline (Pro), histidine (His), isoleucine (Ile), lysine (Lys) leucine (Leu), methionine (Met), cysteine (Cys), valine (Val), phenylalanine (Phe), and tyrosine (Tyr). |
Peptides | Asp-Tyr (Dipeptide) |
Minerals | Macroelements are mostly composed of Potassium (K), phosphorus (P), magnesium (Mg) calcium (Ca), sodium (Na), and phosphorus (P), whereas the majority of microelements mainly included cobalt (Co), copper (Cu), nickel (Ni), manganese (Mn), selenium (Se), iron (Fe) and zinc (Zn) |
Vitamins | Vitamin B, Vitamin C, Vitamin E. |
Phenols | Phenolic acids, p-hydroxybenzoic acid, protocatechuic acid, caffeic acid, catechin, chlorogenic acid, p-coumaric acid, syringic acid, gallic acid, ferulic acid, vanillic acid |
Flavonoids | Apigenin 6,8-di-C-α-L-arabinopyranoside, kaempferide 3-O-α-L-rhamnopyranosyl (1, 6)-β-D glucopyranoside, 6-C-β-D-glucopyranosyl-8-Cα-L-arabinopyranosylchrysin, narcissin, schaftoside, rutin, and 5,7,4′trihydroxy3′,5′dimethoxyflavone. |
Phytosterols | β-sitosterol, campesterol, stigmasterol, cholesterol, ergosterol and stigmastanol |
Glycosides | Taxiphyllin (Cyanogenic glycoside) |
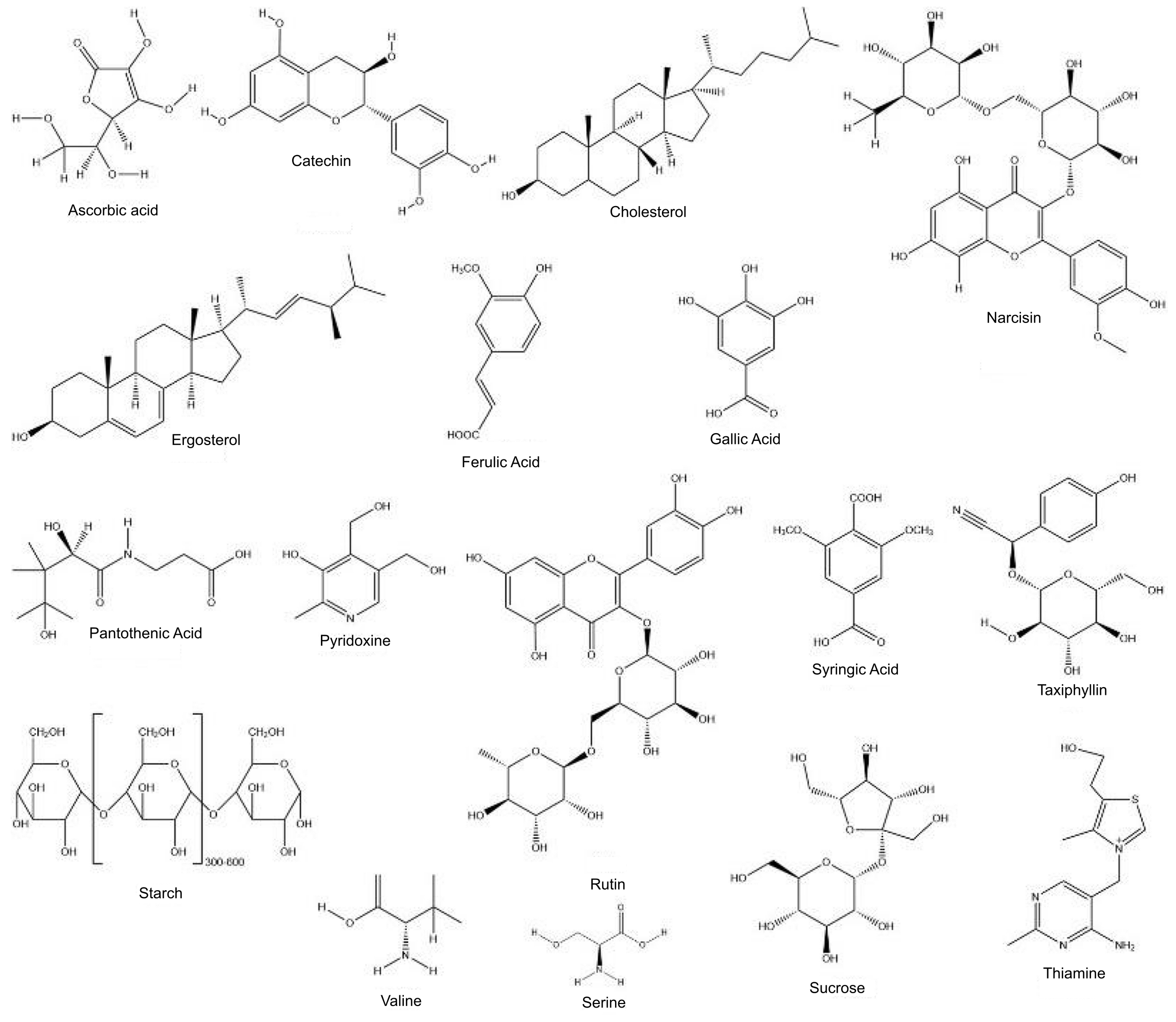
Figure 2. Structures of some phytoconstituents present in B. vulgaris.
In the formaldehyde-induced paw edema method, oral administration of MEBV in graded dosages (100, 200, and 400 mg/kg) resulted in a dose-dependent reduction in paw volume when compared to the control. The oral dose of 400 mg/kg had the considerable effects, resulting in a 46% in paw volume (P <0.01) when compared to the control. The anti-inflammatory activity at this dose was comparable to that of diclofenac (10 mg/kg, p.o.). The maximal anti-inflammatory impact was observed in all dosages of the test medication within 3 hours (27).
In the carrageenan-induced peritonitis model, MEBV decreased peritoneal leukocyte migration at rates of 38, 55.8, and 77.6 percent at doses of 100, 200, and 400 mg/kg, respectively, whereas indomethacin (10 mg/kg) inhibited it at a rate of 60.7%. The neutrophil infiltration was inhibited by MEBV at 32.7, 54.3, and 64.9%, respectively, whilst indomethacin inhibited it by 65.1% (28).
Analgesic Activity
In acetic acid-induced writhing tests, the MEBV demonstrated dose-dependent and substantial analgesic efficacy. The administration of MEBV at dosages of 50, 100, 200, and 400 mg/kg reduced the number of writhing by 25.9%, 29.6%, 37.0%, and 44.4%, in experimental mice when compared to control group. These findings are comparable to those obtained when aspirin was administered to rats at doses of 200 and 400 mg/kg, which resulted in 40.7% and 51.9% reductions in writhing, respectively. Thus, at the highest dose of administration of MEBV 400 mg/kg, exhibited better analgesic activity than aspirin at 200 mg/kg (29).
Antipyretic Activity
The antipyretic activities of B. vulgaris methanol extract has been investigated. The rectal temperature of experimental mice increased 18 hours after Brewer's yeast injection. In the study, a 1000 mg/kg dosage of B. vulgaris methanol extract shown considerable antipyretic efficacy. It produces a temperature drop after 2 hours, and by the end of the 5th hour, the temperatures of the two groups have gone back to normal (30).
Antihyperglycemic Activity
The methanol extract of B. vulgaris (MEBV) has exhibited good antihyperglycemic activity. MEBV, when given at doses of 50, 100, 200, and 400 mg/kg body weight in oral glucose tolerance tests, lowered the quantity of blood glucose in experimental animals’ dose-dependently (30, 31). MEBV reduced blood glucose levels by 8.4, 32.8, 45.8, and 55.3% at these four doses, respectively. However, at a dose of 50 mg MEBV per kg, the results were not statistically significant. Glibenclamide, a common antihyperglycemic drug, lowered blood glucose levels by 50.8% when treated at a dose of 10 mg/kg body weight. Thus, at the highest dose studied, MEBV produced better antihyperglycemic activity to glibenclamide (30).
Antimicrobial Activity
The methanol, ethyl acetate and n-hexane extracts, of B. vulgaris can inhibit the growth of gram-positive and gram-negative bacteria and fungi. These extracts show strong antimicrobial activity against Staphylococcus epidermidis and S. aureus, E. coli, and Aspergillus niger (13, 32). B. vulgaris methanol extract was evaluated against gram-positive, gram-negative, and fungi, in vitro investigation to assess its antimicrobial activity. Among gram-positive bacteria, maximum activity is exhibited against B. subtilis. On the other hand, the highest activity among gram-negative bacteria was seen in E. coli. Inhibition zones, particularly in kanamycin resistance, were reported to be 25 to 35 mm. When compared to the standard kanamycin, the zone of inhibition of the methanol extract was seen to be nearer to the standard (33). This study demonstrates the effectiveness of methanol extracts of B. vulgaris var. Striata against S. aureus and E. coli. It exhibits the largest zone of inhibition against S. aureus (with an average of 13.75 mm and 12.54 mm) and E. coli (with a mean of 8.64 mm and 8.86 12.54 mm) at 12-and 24-hours incubation. The presence of various phytochemicals in all B. vulgaris var. Striata extracts can be attributed for its antibacterial activity (34).
Hepatoprotective Activity
Anghore & Kulkarni (2016) investigated the hepatoprotective effect of the chloroform extract of leaves of B. vulgaris. The carbon tetrachloride-induced hepatotoxicity study in the liver cell of albino rats induces hepatic cell necrosis caused by metabolic activation and production of free radicals from CCl4. The administration of chloroform extract of B. vulgaris was found protective against CCl4-induced increase in enzyme levels of SGOT (Serum glutamic oxaloacetic transaminase), SGPT (Serum glutamic pyruvic transaminase), ALP (Alkaline phosphate) which served as a reliable pathological indicator for jaundice. There is a decrease in enzyme levels of SGOT, SGPT, and ALP which is comparable to the decreases in the standard group. Treatment with 200 mg/kg body weight of chloroform extract of B. vulgaris reduced the elevation of SGOT, SGPT, and ALP. A dose of B. vulgaris extract of 250 mg/kg in albino rats was found to be potential against liver dysfunction and the dose was selected by LD50 for hepatoprotective activity (35).
Antioxidant Activity
Antioxidants can defend organisms from the oxidative stress caused by free radicals (1). According to a recent study, Satya et al., (2009) investigated that fresh shoots of B. vulgaris have a 28.21% antioxidant activity when tested for their ability to scavenge free radicals by the DPPH method (36). The radical scavenging activity of chloroform, acetone, and methanol extract of the leaves of B. vulgaris from the DPPH assay, the IC50 values correspond to 389.23 µg/mL, 300.55 µg/mL, and 262.90 µg/mL respectively (37).
Antimalarial Activity
The antimalarial effect, and chemopreventive capacity of aqueous leaf extract of B. vulgaris in malaria parasitized mice was investigated. A total of 30 male mice, grouped into six (n = 5), was used. The results obtained showed that B. vulgaris is rich in flavonoid (262.08 µg CE/g) and phenol (0.91 g AAE/ 100 g). There was significant reduction on the activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and Gamma glutarmyl-transferase (GGT) upon treatment as compared with the control groups (p < 0.05). Concentration of total bilirubin (TB), direct bilirubin (DB) and serum electrolytes (sodium, calcium, phosphorus and chloride) decreased in treated groups; serum urea, creatinine and uric acid also reduced significantly as against the control groups (p < 0.05). The hepatoprotection and renal function restoration observed upon the administration of the plant extract indicate to a far reaching end that B. vulgaris leaf extract would be a promising natural antimalarial product devoid of side effects upon use, especially when administered within the dose range of 100 – 200 mg/Kg body weight investigated in this study.
Anticonvulsant Activity
The extract of leaves of B. vulgaris was investigated for anticonvulsant potential. Adebayo et al. studied the effect of the extract on the pentylenetetrazole-induced convulsion model and found at 100 mg/kg, 200 mg/kg, and 400 mg/kg exhibited (p < 0.05) prolongation of death time and offered 60%, 80%, and 100% protection respectively compared to the control group (10 mL/kg) which offered 0% protection. The dose of 400 mg/kg elongated the onset of clonic, tonic convulsions, and death latency (39).
Antiamnesic Activity
The methanol extract of the leaves of B. vulgaris was studied for antiamnesic activity by scopolamine-induced amnesia on the Y-maze task. Scopolamine significantly (p < 0.05) reduced the percentage of correct alternation on the Y-maze when compared to the control-treated group on percentage alternation on the Y-maze task. However, when compared with the control-treated group, B. vulgaris extract significantly (p < 0.05) in a dose-dependent pattern increased the reduced alternation induced by Scopolamine. Piracetam, a positive control drug significantly (p < 0.05) reversed the reduced alternation induced by Scopolamine in mice (39, 40).
Antivirus
The ethanol extract of B. vulgaris was analyzed for its antiviral activity against three human viruses: measles, yellow fever, and poliovirus with standard laboratory tests of which the extract of B. vulgaris produces inhibition only for the measles virus at MIC 62.5 μg/mL (41).
Conclusion
Bamboo has been used for centuries as a food source and to treat a variety of illnesses. It significantly affects people's socioeconomic well-being. Numerous research has evaluated the plant's potential as a medicine. Nevertheless, there is still a need for in-depth research on bamboo, outside of its application in food and crafts. Bamboo's ethnopharmacological uses must be backed up by substantial academic research before they can be widely used in a range of therapeutic procedures. Due to their high quantity of beneficial proteins, amino acids, carbs, and other essential minerals and vitamins, as well as their extremely low-fat content, they also have a significant potential for usage as crucial health foods.
Declarations
Ethics Statement
Not applicable.
Data Availability
Not applicable.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Fitri, A., Asra, R., & Rivai, H. (2020). Overview of the traditional, phytochemical, and pharmacological uses of gold bamboo (Bambusa Vulgaris). World Journal of Pharmacy and Pharmaceutical Sciences, 9(8), 21.
- Sangeetha, R., Diea, Y. K. T., Chaitra, C., Malvi, P. G., & Shinomol, G. K. (2015). The amazing bamboo: a review on its medicinal and pharmacological potential. Indian J Nutr, 2(1), 1-7.
- Sharma, Y. M. L. (1980). Bamboos in the Asia Pacific Region. In Bamboo research in Asia: proceedings of a workshop held in Singapore, 28-30 May 1980. IDRC, Ottawa, ON, CA.
- Rubatzky VE, Yamaguchi M (1997) World vegetables: principles, production and nutritive values. Chapman and Hall, New York, pp 658–660
- Lobovikov, M., Paudel, S., Ball, L., Piazza, M., Guardia, M., Wu, J., & Ren, H. (2007). World bamboo resources: a thematic study prepared in the framework of the global forest resources assessment 2005 (No. 18). Food & Agriculture Org.
- GEMCO energy (2022). Make bamboo pellets. http://www.gemcopelletmills.com/make-bamboo-pellets.html
- Banerjee, S., Basak, M., Dutta, S., Chanda, C., Dey, S., Dey, A., Somkuwar, B. G., Kharlyngdoh, E., & Das, M. (2022). Sustainable uses of bamboo by indigenous people with special emphasis on North-East India. Indigenous People and Nature, 543-576. https://doi.org/10.1016/B978-0-323-91603-5.00016-6.
- Hammer, K. A., Carson, C. F., & Riley, T. V. (1999). Antimicrobial activity of essential oils and other plant extracts. Journal of applied microbiology, 86(6), 985-990.
- Mandal, V., Mohan, Y., & Hemalatha, S. (2007). Microwave assisted extraction—an innovative and promising extraction tool for medicinal plant research. Pharmacognosy reviews, 1(1), 7-18.
- Larson, R. A. (1988). The antioxidants of higher plants. Phytochemistry, 27(4), 969-978.
- Parekh, J., & Chanda, S. (2007). In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkish Journal of Biology, 31(1), 53-58.
- Coffie, G. Y., Antwi-Boasiako, C., & Darkwa, N. A. (2014). Phytochemical constituents of the leaves of three bamboo (Poaceae) species in Ghana. Journal of Pharmacognosy and phytochemistry, 2(6).
- Owolabi, M. S., & Lajide, L. (2015). Preliminary phytochemical screening and antimicrobial activity of crude extracts of Bambusa vulgaris Schrad. Ex JC Wendl.(Poaceae) from southwestern Nigeria. American Journal of Essential Oils and Natural Products, 3(1), 42-45.
- Karanja, P. N. (2017). Physicochemical properties of bamboo shoots of selected species grown in Kenya and utilization as human Food (Doctoral dissertation, Faculry of Agriculture, JKUAT).
- Lin, Z., Chen, J., Zhang, J., & Brooks, M. S. L. (2018). Potential for value-added utilization of bamboo shoot processing waste—recommendations for a biorefinery approach. Food and bioprocess technology, 11(5), 901-912.
- Oshima, Y., Watanabe, T., Endo, S., Hata, S., Watanabe, T., Osada, K., & Takenaka, A. (2018). Effects of eicosapentaenoic acid and docosahexaenoic acid on anxiety-like behavior in socially isolated rats. Bioscience, biotechnology, and biochemistry, 82(4), 716-723.
- Christian, A. L., Knott, K. K., Vance, C. K., Falcone, J. F., Bauer, L. L., Fahey Jr, G. C., ... & Kouba, A. J. (2015). Nutrient and mineral composition during shoot growth in seven species of P hyllostachys and P seudosasa bamboo consumed by giant panda. Journal of Animal Physiology and Animal Nutrition, 99(6), 1172-1183.
- Nirmala, C., Bisht, M. S., Bajwa, H. K., & Santosh, O. (2018). Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends in Food Science & Technology, 77, 91-99.
- Pandey, A. K., & Ojha, V. (2013). Standardization of harvesting age of bamboo shoots with respect to nutritional and anti-nutritional components. Journal of Forestry Research, 24(1), 83-90.
- Thapa, N., Lamichhane, J., & Gauchan, D. P. (2018). Phytochemical, antioxidant, antimicrobial and micropropagation study of Bambusa species. Int. J. Res, 5(21), 77-88.
- Liu, H., Zhang, C., Liu, Y., & Duan, H. (2019). Total flavonoid contents in bamboo diets and reproductive hormones in captive pandas: exploring the potential effects on the female giant panda (Ailuropoda melanoleuca). Conservation physiology, 7(1), coy068.
- Ogbe, R. J., Ochalefu, D. O., Mafulul, S. G., & Olaniru, O. B. (2015). A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J. Plant Sci. Res, 5(4), 10-21.
- Nartey, F., Smith, R., & Bababumni, E. (1980). Toxicological aspects of cyanogenesis in tropical foodstuffs. Toxicology in the Tropics, 53-73.

 ETFLIN
Notification
ETFLIN
Notification







