Cold Atmospheric Plasma: A Noteworthy Approach in Medical Science
by Bedanta Bhattacharjee ★ , Rajashri Bezbaruah , Damanbhalang Rynjah , Arzoo Newar , Sindhuja Sengupta , Padmanath Pegu, Nikita Dey, Shekhar Chandra Bora , Dhunusmita Barman
Academic editor: Abd. Kakhar Umar
Sciences of Pharmacy 2(2): 79-103 (2023); https://doi.org/10.58920/sciphar02020046
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
25 Mar 2023
11 May 2023
08 May 2023
11 May 2023
Abstract: Cold atmospheric plasma (CAP) is a novel technology with boundless significance that can be used in the medical sector that offers noninvasive in-vivo applications without damaging the living tissues. CAPs can be obtained by curtailing the concentration of high-energetic electrons per phase and by freezing molecules/atoms (devoid of charge) in plasma utilizing gas circulation and atmospheric air, which includes a variety of charged and neutral reactive entities, UV rays, electric currents, and fields, etc. that have an influence on cellular material in a multitude of diverse manners. Reactive oxygen species (ROS) and reactive nitrogen species (RNS), produced by the plasma, essentially cause biological and therapeutically advantageous plasma effects. CAP plasma has several important biological functions, including the deactivation of pathogens, induction of tissue restoration and cell propagation, the annihilation of cells by triggering apoptosis, etc. Several fundamental concepts are defined, even if the precise process of the effect of plasma on biomolecules is still not properly identified. Depending on the biological synthesis of RNS and ROS in reactions to plasma emissions, the present review described several aspects of plasma therapy in neuroscience, particularly in anti-glioblastoma, neuro-differentiation, and neuroprotection and also the various applications of CAP in medical fields where it is used in the therapy of SARS-CoV-2, cancer therapy, and chronic and acute wounds. Furthermore, the proliferation in stem cells, dental medicines, dermatology, and a brief insight into CAP devices and their risk factors was highlighted.
Keywords: CAPPlasmaCancerSARS-CoV-2Wound healingCAP devices
Introduction
Plasma is the fourth kind of matter in physics, after solid, liquid, and gas, originating in a powerful electronic field. The resultant partially ionized gas is composed of an extremely conductive combination of electrons and ions, excited species, reactive molecules, UV radiation, and electric fields. It is generally preceded by massive heat generation. This warmness is released by interactions of electronically developed high electrons and gas molecules associated with excitation, ionization, and disintegration actions. Previously plasmas have been used in healthcare for several decades for decontamination, coagulation, or else cauterization, as well as in cosmetic medicine (1). Plasma is categorized into non-thermal, high-temperature, or thermal (2). All particles in the high-temperature plasma (heavy particles and electrons) have a comparable heat, indicating that they are thermally equilibrated. The thermal (quasi-equilibrium plasma) is the sole domain of thermal equilibrium inside the plasma. Ultimately, non-thermal-non-equilibrium plasma contains components that are not thermally balanced, and this plasma is known as "cold plasma” (3).
Moreover, such plasmas are typically developed in a vacuum or are exceedingly hot, making them unsuited for use in living cells. Recent advances in plasma research have created cold atmospheric plasmas (CAPs) that achieve atmospheric parameters and offer noninvasive in-vivo applications without damaging the tissue. Here, 'Cold' denotes that the plasma devices are generally operated at temperatures lower than 40°C or that the administration of plasma is in a pulsed manner, eliminating heating of the tissue in a specific deployment interval. CAPs can be obtained by curtailing the concentration of high-energetic electrons per phase (for surface micro discharge [SMD] devices, 1 kilohertz [kHz] is needed) and by freezing molecules/atoms (without a charge) in plasma utilizing gas circulation and atmospheric air (e.g., in MicroPlaSter torches [Adtec Plasma Technology Co. Ltd., London, UK]) (4). The effectiveness of CAP is attributed to its numerous constituents, like reactive oxygen and nitrogen species (RONS), that demonstrate beneficial action in biological and economic contexts (5, 6). Among the several strategies for producing cold plasma under atmospheric parameters, two primary aspects of CAP devices dominate clinical and preclinical exploration in plasma therapies: plasma jets and dielectric barrier discharges (DBD) (7). DBDs are distinguished by igniting plasma in a void among an insulated electrode of high voltage and the subject to be addressed (volume DBD) or within an individualized electrode structure approach (e.g., grid-like or circular) that is insulated from a counter electrode (surface DBD). In the hindmost situation, the active plasma has no immediate connection with the subject to be medicated. The operating gas for plasma production in DBDs is typically ambient air (8). Even though CAP is useful in lowering a microbiological load, it lacks the sterilization and disinfecting characteristics of low-pressure plasma (9). Remarkably, CAP's milder effects allow for immediate administration to tissues and cells. Its capability to lower microbial strain makes it a viable choice for replacing antibiotics and combating antibiotic-resistant strains of bacteria (10). Another significant benefit of CAP-generating systems is their cheap production rates. As a result, affordable, effective, and less expensive CAP devices will almost certainly lessen the cost burden on the healthcare budget by conventional treatments (4). One notable merit of these CAPs is that they preserve their virucidal, antifungal, and antibacterial (sporicidal) activity (11). Such aspects appeal to a transformative futuristic technique when tolerance seems more difficult, particularly in light of global sanitation issues caused by increased microbial resistance (12). In this review, we will focus on the various applications of CAP in medical fields where it is used in the therapy of SARS-CoV-2, neurological complications, cancer therapy, and chronic and acute wounds. Furthermore, the proliferation in stem cells, dental medicines, and dermatology. Also, a brief insight into CAP devices and their risk factors was highlighted.
Techniques of CAP Production
Low applied electric field and high electron-neutral collision frequency make producing non-thermal plasma at atmospheric pressure difficult. The good news is that numerous strategies have emerged throughout the years to address challenges. As mentioned, cold plasma is created in the open using various procedures. Some examples include the corona discharge, atmospheric pressure plasma jets, and dielectric barrier discharges. CAP plasma may be created using multiple working gases, including nitrogen, argon, helium, heliox (helium and oxygen mixture), air, etc. This section provides a high-level summary of the most popular methods for producing plasma in CAPs.
Dielectric Barrier Discharge (DBD)
The DBD, which employs an alternating or pulsing electric field, is a popular method for producing CAP plasma. As the name suggests, one of the two electrodes used to create the discharge must have a dielectric cover. The dielectric layer's job is to reduce the discharge current and prevent the spark or arc transition (13). Due to the lack of noise during discharge, DBDs are often referred to as "silent" discharges. In a DBD, the electrode gap is typically between 0.1 mm and several centimeters long. DBDs use various dielectric materials, including glass, quartz, ceramics, polymers, etc. Discharge current must be insulated by a dielectric layer with a high enough breakdown strength to prevent sparking or arcing. A thicker layer, however, calls for a larger voltage. Therefore, a trade-off is required.
In most cases, a chamber is built around the electrode setup, so different gas mixes may be introduced in the space between the electrodes (14). DBDs are often used in industrial settings, driven by high-voltage sources operating at kHz. DBD may be set up in various ways, but the core idea remains the same. They can be flat, parallel plates divided by a cylinder or a dielectric, or coaxial plates comprising a dielectric tube in between them. Recently, a DBD with floating electrodes was created by Fridman et al. Just like the original DBD, this one has two electrodes: an active electrode and a high-voltage insulated electrode (15). The second electrode of floating electrode-DBD (FE-DBD) is not grounded, setting it apart from DBD (16). You may use human skin, a sample, or anything else for the second electrode. The powered electrode must be relatively near the second electrode's surface to trigger the discharge.
At ambient pressure, a DBD's discharge is often non-uniform filamentary, which may lead to uneven sample treatment. The dynamic dispersion of these filaments determines the discharge's visual appearance. However, DBDs may generate diffuse, homogenous plasma rather than filamentary, provided the right circumstances are met (17). Many research groups have successfully created diffuse, homogeneous, atmospheric pressure glow DBD plasmas. A Townsend breakdown is started to create a glow DBD instead of a streamer breakdown. For an avalanche to occur in a weaker electric field and prevent the proliferation of positive space charges, enough initial seed electrons must be in the gap before breakdown. The remaining species of the first half of the given voltage phase in DBDs give the seeding electrons or elevated initial field for the subsequent discharging process. This phenomenon is called the "memory effect” (16). Figure 1 represents a few popular DBD electrode designs.
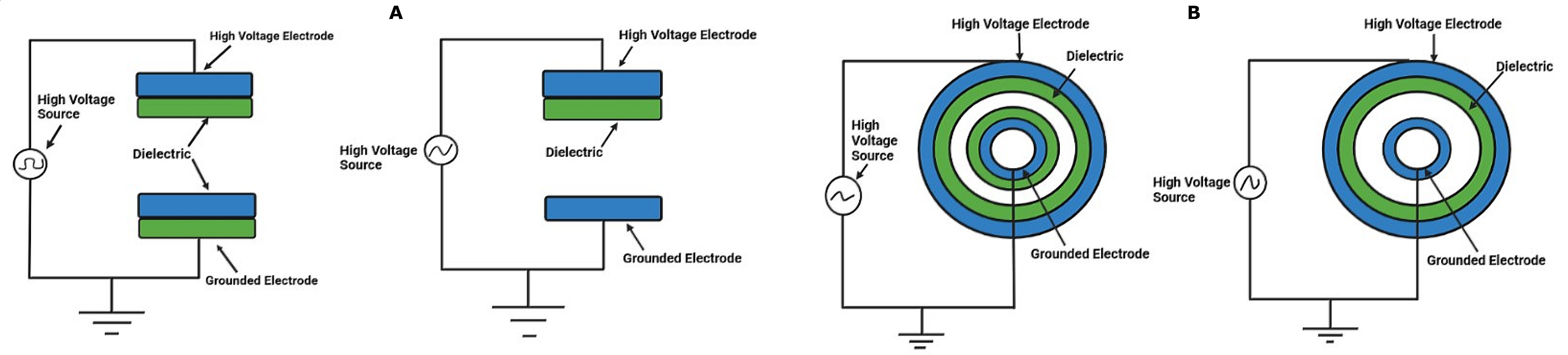
Figure 1. DBD diagrams with multiple electrode arrangements of (A) Planar DBD and (B) Cylindrical DBD.
Atmosphere Pressure Plasma Jet (APPJ)
Jets of atmospheric pressure plasma (non-thermal) are among the most adaptable methods for producing CAP in medicine. Since the plasmas they generate are not limited in any way by the electrodes, they may be utilized for direct therapy on objects of any form or size. Therefore, they can transport the necessary active short-lived radicals and charged particles the plasma creates to the treated material. Various plasma jet configurations are documented in the literature as producing CAP plasma (18). There are several different ways to categorize CAP plasma jets. A few writers have separated CAP plasma jets into categories based on the excitation frequency of the power sources. This frequency spectrum covers the whole range from DC to GHz. In keeping with their respective mechanisms, we have a pulsed-dc plasma jet, single-electrode (SE) jets, Microwave driven plasma jet, DC plasma jet, RF-operated plasma jet, KHz-operated plasma jet, dielectric-barrier-discharge (DBD) jet, Dielectric-free-electrode (DFE) jets, and DBD-like jets are the four main categories.
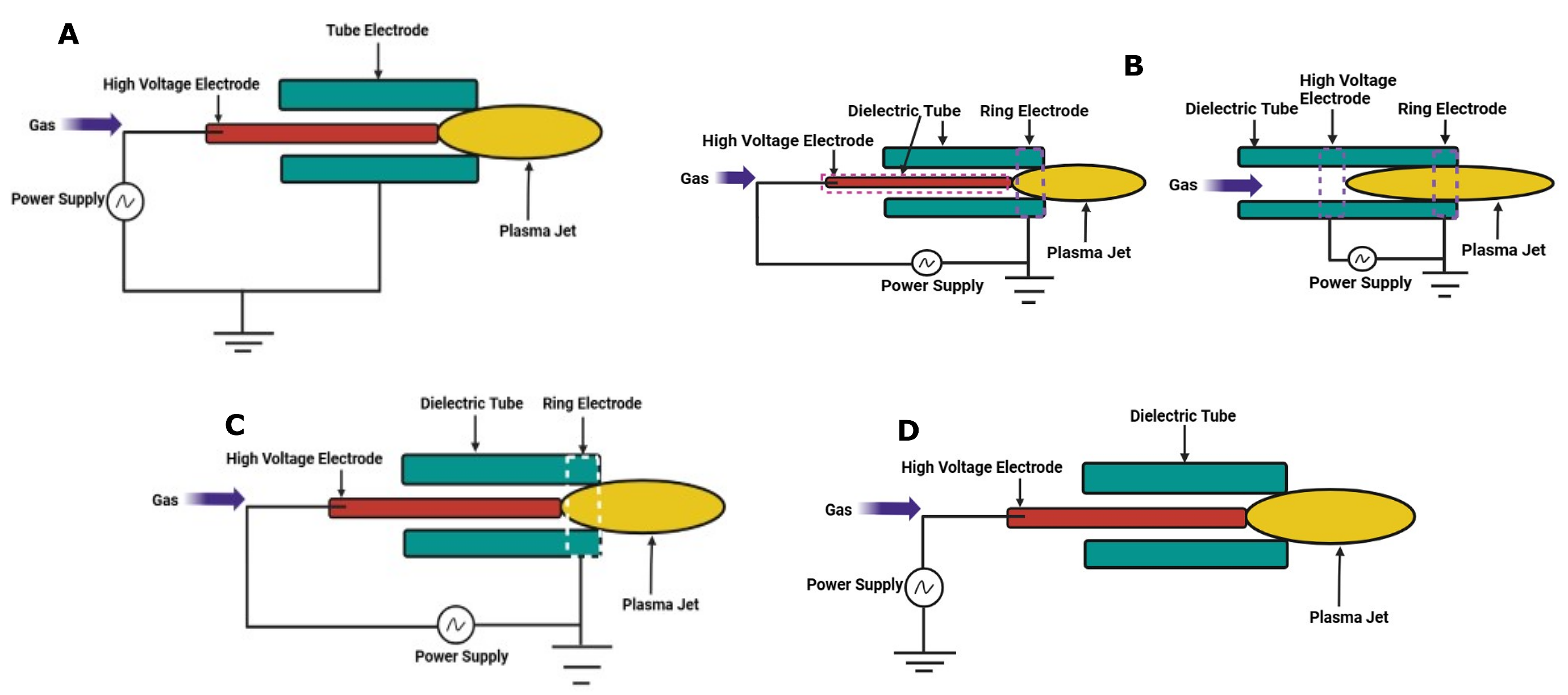
Figure 2. Common electrode arrangements of (A) DFE jet, (B) DBD jet, (C) DBD-like jet, and (D) SE jet.
Figure 2 (a) shows that each DFE jet comprises an internally powered electrode and an outer grounded electrode. There is nothing to act as a dielectric between the two electrodes. This jet has a relatively high gas temperature, necessitating cooling water to ensure its continued functioning. Arcing is always possible if the jet's normal operating circumstances are not satisfied. Direct medicinal uses of the DFE jet are not feasible. Radiofrequency (RF) energy is used to power it (19). The plasma in DBD jets is not in touch with either electrode due to the presence of a dielectric layer in between them. This plasma jet has a shallow energy requirement (of a few watts). Arcing with these plasma jets is not dangerous, making them perfect for use in biomedical settings. Inputs of kHz alternating current and pulsed direct current may power the DBD jets. Common electrode arrangements for DBD jets are seen in Figure 2 (b). When plasma is not in touch with anything, the discharge of DBD-like plasma jets looks quite similar. No insulating medium separates the active electrode from the treated substance. The plasma in these jets may be quite reactive and can receive much more power than conventional plasma accelerators. Such jets have their benefits if arcing can be prevented, and for safety reasons, these gadgets need special caution when used in the medical field as they are powered by kHz ac, RF, or pulsed dc sources. Figure 2 (c) depicts the standard setup of a DBD-like plasma jet (20). A key difference between SE and DBD-like jets is the absence of an electrode outside the dielectric tube. These jets are compatible with dc, kHz ac, RF, and pulsed dc power. The possibility of an electric spark makes these jets unsuitable for use in the medical field. The fundamental electrode setup of a SE plasma jet is shown in Figure 2(d). When seen with rapid photography, the plasma generated by CAP plasma jets reveals its true discrete character, despite its apparent homogeneity to the naked eye. There is a "bullet"-like structure in the plasma volume, and it travels at more than 10 km s-1. Lu et al. used a pulsed-dc plasma jet and Teschke et al. used an RF-driven plasma jet and were the first to report the plasma jet's discrete character (21, 22).
Corona Discharge Tube (CDT)
In a corona discharge, the electrical field is strongest close to the edge-electrode and rapidly diminishes with further distance, indicating a non-equilibrium state. Due to the very localized nature of the electrical field, the gas fragments down in the vicinity of the sharp electrode (23). A conductive zone is formed, but the electric field is deficient in triggering an electrical fragmentation in neighboring items. As can be seen in Figure 3, an asymmetric electrode pair configuration, such as wire-to-cylindrical electrodes or point-to-plane, may produce this sort of nonuniform electric field. Depending on the HV electrode's polarity, two distinct forms of corona discharge exist. Different from one another in terms of physics is the corona discharge, which may be either positive or negative. The huge disparity in mass between electrons and ions causes this effect. Electrons should be pulled to the HV electrode for the corona to be positive, whereas positive ions must be deflected. The gas close to the electrode is ionized by light, producing secondary electrons. Attracting the electrons to the electrode sets off a chain reaction involving the electrons' inelastic collision with neutral gas molecules (20). Contrarily, emission from the HV electrode occurs in the negative corona. Here, the photoelectric action from the electrode surface is principally responsible for producing the secondary electrons.
A Townsend analysis, or something quite similar, might describe this procedure. Ionization from the collision then causes the avalanche of electrons to multiply. Negative ions accumulate as we move away from the electrode, and the electric field weakens. So, there is less ionization in such areas. Powering a discharging corona may be pulsed voltage, alternating current (AC), or direct current (DC). Medical surface preparation, bacterial inactivation, material processing, electrophotography, water purification, copy machine, wound healing, ozone synthesis, etc., are only some of the many sectors it finds use. This plasma allows for great versatility in treating a wide range of medical equipment and materials, such as pill bottles, surgical gowns, catheter tubing, syringe barrels, IV tubes, etc. (20, 23).

Figure 3. Common electrode arrangements of (A) Point to Plane and (B) Wire to cylinder arrangement.
Application of CAP for Therapy of SARS-CoV-2
A new viral disease called coronavirus disease 2019 (COVID-19) is brought on by coronavirus 2 and results in severe acute respiratory syndrome (SARS-CoV-2) (24-26). A very rare pandemic was brought on by SARS-CoV-2, and investigations revealed that the virulent pathogens could survive for several periods on various exterior surfaces (such as metal, cardboard, and plastic) (27, 28). People are at great risk of contracting SARS-CoV-2 from contaminated surfaces, so it's crucial to stop the transmission cycle by finding new inactivation techniques. Cold atmospheric plasma (CAP) functioning at atmospheric pressure and ambient heat can securely and efficiently cure contaminants on a uniform and intricate surfaces (29, 30). Reactive oxygen and nitrogen species (RONS) are one of the numerous parts of CAP that demonstrate positive behavior for biological and industrial uses and a reason for the substance's effectiveness (31-33). In a recent investigation, Chen et al. used CAP management to render SARS-CoV-2 inactive on various surfaces, including metal, plastic, cardboard, and composite leather used in basketballs, baseballs, and football (31). CAP has tremendous potential as a secure and efficient way to stop the spread of viruses and diseases on various surfaces that frequently come into contact with people.
Surfaces with virus contamination were subjected to helium or argon plasma several times. Surfaces that were infected with the virus but weren't in contact with plasma were also used as a control. SARS-CoV-2 specimens containing the virus were extracted from surfaces using 100 µL of DMEM culture medium. In a 96-well system, the incidence of the collected SARS-CoV-2 specimens was determined. Each collected specimen was sequentially diluted 10 times before 100 µL of diluted viral injection was introduced to the plate. The plates were evaluated for evidence of a viral cytopathogenic impact (CPI) three to four days after infection. The plates that tested negative for CPI at the minimum viral concentration were detected and considered in determining the virus intensity for each specimen (34). Vero-E6 cells infected with SARS-CoV-2 have a viral cytopathic impact. Argon (Ar)-fed CAP therapy was seen to inactivate every instance of SARS-CoV-2 on the surfaces in less than 3 minutes. More precisely, after 30 seconds of exposure, metal surfaces showed signs of decontamination. Most data points on the football field made of plastic and leather demonstrated virus deactivation for half and one-minute treatments. For a 60-s treatment, surfaces like cardboard and basketballs effectively inactivated viruses; few data points show this impact for 30-s treatments. Additional tests revealed that cotton fabric used in face masks similarly inactivated viruses. It was discovered that material composition, roughness, and absorptivity are three crucial factors in the surface deactivation of SARS-CoV-2 through CAP. The authors found that even at 300 seconds, Helium (He)-fed plasma did not completely eradicate SARS-CoV-2 on metal and plastic surfaces, in contrast to Ar-fed plasma. This is probably because He-fed plasmas had significantly lesser RONS levels under the same operating conditions than Ar-fed plasmas. At exposure periods of 250 and 5000 ms, the optical emission spectrum was used to detect Ar-fed and He-fed plasma jets. As a result, RONS concentration significantly impacts SARS-CoV-2 inactivation (35).
Application of CAP for Therapy of Neurological Complications
Any condition affecting the brain, peripheral nervous system, or vegetative nervous system is referred to as a neurological disorder, such as trauma, spinal damage, traumatic brain injury, and Parkinson's and Alzheimer's (36). Central nerve tissue (CNS) transplantation has emerged as one of the foremost intriguing therapies for neurotraumatic and neurological disorders due to the advancement of cloning techniques and the extraction of neural stem cells (NSCs). These procedures have inadequate selectivity of a certain cell type differentiation and the potential to produce chemical toxicity and glial scarring following transplantation. Since the advent of CAPs two decades ago, plasma medicine has gained much attention from researchers worldwide (37). In the current research, Jang et al. demonstrated that a CAP relying on a DBD effectively triggered in-vitro and zebrafish neural progression. The findings offer hope for managing neurological dysfunction in the upcoming years (38).
Furthermore, they explored the molecular and chemical interactions between the CAP circuit and the ERK/Ras/Trk signaling system responsible for neuronal development. In this work, the in-vivo analysis was conducted using a recombinant zebrafish (Danio rerio) embryo and murine neuroblastoma-derived cell line, i.e., Neuro 2A (N2a). A DBD plasma was used for the therapy, with an operating gas combination of O2 and N2 and a power input of 1 Watts. Following 24 hours of photonic therapy, the diameter of the CAP-treated N2a cells was greater than 4-fold that of the naive cells, with a maximal nerve length of almost 70 mm and a mean of 46.3 ± 1.5 mm. The dopaminergic (DA) nerve cells, the main origin of dopamine in the CNS and significant in regulating numerous neural mechanisms, were part of the NSCs that undergo terminal proliferation to become developed nerve cells. In addition, Parkinson's disease is closely linked to the loss of DA neurons (38). Recombinant zebrafish embryos challenged to a 1 min CAP exposure in-vivo revealed that the in-vitro findings are also biologically intriguing. Green fluorescent protein (GFP) is exclusively expressed in postmitotic mature nerve cells. After an incubation period of 6 hours, GFP activity continued to elevate and remained there for as long as 33 hours. Following 36 hours of post-fertilization, embryos were subjected to CAP, and GFP+ developed nerve cells were apparent in the CNS of maturing zebrafish within 6 hours. A 1.17-time rise in developed GFP+ nerve cells was reported in later-stage embryos exposed to CAP.
Concerning the physiological methodology of the plasma therapy, the researchers revealed that NO acted as an arduous extracellular messenger. In contrast, cytosolic H2O2 and mitochondrial O2 collectively served as messengers of ROS that played important functions in interacting with cells throughout CAP-mediated neural proliferation (38). Another research team used a micro-plasma jet device as a separate CAP source to successfully induce NSCs differentiation in-vitro in a related investigation (39). In previous work, primary rat NSCs and C17.2 immortalized neural progenitor cell lines demonstrated fast and efficient NSC maturation. Moreover, the research by Jang et al. improved neural maturation in rodents to a particular type of developed nerve cells, which is a significant breakthrough for the upcoming use of CAPs to alleviate neurological conditions. CAP-enhanced NSC and progenitor cell maturation have a lot of rewards over conventional chemical approaches that use serum deprivation, resveratrol, or retinoic acids. This novel observation demonstrates that maturation using CAP was 2-3 fold quicker and proved 2-2.5-time improved maturation effectiveness.
Interestingly, DA nerve cells, which are specifically needed in the management of Parkinson's disease, compose 70% of the developed nerve cells which CAP induces. Additionally, it is simple to manipulate plasmas by altering the plasma source tool, operating gas, input power, or electrical power source. Plasma dose significantly impacts the biological outcomes that result from plasma: overall, a small plasma dose can promote cell motility, maturation, and multiplication, whereas excessive plasma dose results in apoptosis. CAPs have indeed been reported to be safe for treatment cells in both in-vivo and in-vitro (40). Hence, this rapid, predictable, single-step, effective procedure for accelerated and targeted neural cell maturation will be a plausible potential therapeutic approach for neurological dysfunction.
Application of CAP for Therapy of Cancer
A potential approach for more successful cancer therapy is CAP. However, its ultimate impact on cancer cells is intriguing. Higher ROS and RNS production by cancer cells can lead to apoptosis even if it enhances their proliferative potential (41). Then, CAP raises this to a point where the cancer cell is killed. Healthy cells should be able to withstand this increase in damage due to the breakage of double strands and alterations in their antioxidant system (42). The cancer cells also contain more aquaporins, making it easier for ROS and RNS to enter the cell (43, 44). The lipid composition of the membrane can also affect how much ROS and RNS diffuse into the cell (45). Because cancer cells often have lower cholesterol levels, they are more vulnerable to peroxidation (46). The cell membrane then develops larger perforation due to membrane lipid peroxidation, facilitating ROS and RNS to penetrate more freely (47). An overview of the pathway's underlying function highlights that the administration of CAP to cancerous cells triggers a series of biological reactions. The main factor starting cancerous cell death is this rise in radical intermediates. DNA and mitochondrial dysfunction, cell death, cell cycle disruption, growth arrest, and immunogenic induce apoptosis are the particular reactions in this case (48). However, the real outcome is dose-dependent (49, 50). In a pilot study, Keidar et al. evaluated CAP's efficacy in treating tumors (51). The healthy cell lines stayed adherent. However, they originally reported that skin carcinoma cell lines detached from the growth medium following CAP administration, which lowered their numbers (51). Kaushik et al. subsequently investigated the plasma release action on the MRC5, HEK293, A549, and T98G cell lines to reveal the influence of ROS on tumor cell fatality (52).
Evaluating the effectiveness of cancerous cell lines over non-cancerous cell lines, the researchers demonstrated that HEK293 and MRC5 were not significantly altered. Plasma release of ROS and H2O2 changed the state of the mitochondrial matrix. As a result, the intrinsic apoptotic cascade was activated, raising the overall activity of the pro-apoptotic gene and decreasing the expression of anti-apoptotic genes. There was also a shift in the protein level of MAPK/ERK1/2 cellular signaling function. With ROS scavengers, the resulting impact can be prevented. Normal cells and cancerous cells have different metabolic properties. Specifically, nucleic acid, protein, and lipid macromolecules are formed when initiating changes in tumor cells and integrating simple carbons. Therefore, secondary metabolites are produced, which cancerous cells might utilize for development and multiplication (53). CAP outcomes on abnormal cancerous cell metabolic processes were investigated by Xu et al. (54). Leukemia cells displayed distinct glutamate, aspartate, and alanine metabolism based on the GC-TOFMS (Gas Chromatography-Time of Flight Mass Spectrometry) and KEGG (Kyoto Encyclopedia of Genes and Genomes) investigations.
Moreover, following CAP therapy, the researcher reported decreased glutaminase function in tumor cells. As a result, less glutamine is metabolized to glutamic acid. A glutamic acid deficit associated with glutamine aggregation can hinder leukemia cells from multiplying and potentially induce apoptosis (54). The advantages of CAP devices, including their anti-tumor effectiveness, have been consistently shown in research on experimental animals and cell lines. Glioblastoma cell lines reduced survivability following plasma therapy, according to research. This investigation also showed that the temozolomide (an alkylating drug) resistant cell lines restored their responsiveness (55). Additionally, several publications have claimed that CAP treatment led to different cancerous brain cell lines losing survivability and inducing cell death (56).
Primary lung carcinoma TC-1 cell lines have observed apoptotic cell death following plasma release. Although to a much smaller degree, this has additionally been seen in fibroblast cell lines (57). The proposed idea of reducing the footprint of plasma tools to facilitate simpler CAP application in the deepest structures and lesions was, nevertheless, the study's significant contribution. Here, plasma release produced by apparatus ranging in diameter from 125-440 µm was responsible for inducing cell death. µCAP tools were best applied to glioblastoma cell lines in-vitro and mice with brain tumors (58). In the second case, the plasma release out of a 70 µm tool raised the ROS and RNS, significantly decreasing the survival of U87MG glioma cells. In addition to applying plasma discharges into an intracranial endoscopic channel to mouse brains, this technology was indeed capable of suppressing cancer progression (58). The tool variables were then adjusted by the researchers for such most effective treatment of breast and brain malignancies (59). When 4T1 breast cell lines were subcutaneously introduced into experimental animals, the in-vivo plasma action was shown to exist. Plasma discharge from a CAP tool with a diameter of 250 µm was applied to the mouse body malignant expansion derived from such cell lines. Interestingly, plasma delivery reduced cancer growth for 3 minutes, equivalent to treatment. The proportion of pro- to anti-apoptotic gene expression at the cellular stage also saw a dramatic alteration also changed significantly (60). Mashayekh et al. investigated the action of CAP on melanoma B16/F10 cell lines in-vitro and in-vivo in treated mice (61). Their research showed that most cell lines lost survival and that the malignancies in animal models significantly compressed. After receiving 3 minutes of CAP therapy, cell-line outcomes were shown in 48 hours, and in-vivo tumor compress was on par with those seen with cancer therapy. In conclusion, because plasma is most conveniently delivered access to the cutaneous structure, evaluations of CAP impacts on skin carcinoma therapies were carried out before. For instance, following CAP treatment, G361 malignant cells reduced vitality and separated from the interface. Reduced focal adhesion kinase (FAK), integrin activation, and modified actin filament architecture were present in such cells. This finding supports the theory that connections between extracellular matrix (ECM) and integrin may contribute to plasma-mediated apoptosis (62).
Table 1. Overview of in-vitro tumor therapeutic uses for CAP.
|
Gas Infused/Plasma Tool |
Type of cancer cell line |
Exposure Time |
Ref(s) |
|
Kinpen® MED; O2+He/Plasma jet; He/Plasma jet |
Head and neck cancer cell lines |
20-150 s; 10-45 s |
|
|
Plasma micro-jet; Plasma jet |
Cervical cancer cell lines |
10-15 s; 5 min |
|
|
Ar/NEAPP jet tool |
Gastric cell lines |
5 min |
(78) |
|
Ar/Plasma jet; He/Plasma gun; Ar/Plasma jet |
Pancreatic cell lines |
3 min of solution therapy; 10-90 s; 30 s- 5 min |
(79) |
|
DBD tool; He/Plasma micro jet; He/Plasma jet |
Melanoma cell lines |
40 s; 15 s; 30 s |
|
|
He/Plasma jet; Ar+He/Plasma jet; O2+He/Plasma torch; FE-DBD tool |
Colorectal cancer cell lines |
5-30 s; 60-120 s; 1-4 s |
|
|
He/Plasma pencil |
Leukemia cell lines |
10 s-10 min |
(82) |
|
Ar/Plasma jet; He/Microplasma jet tool |
Lung cancer cell lines |
3 min of liquid therapy |
(83) |
Utilizing plasma-containing nanoparticles coupled to the anti-FAK antibodies, it became feasible to specifically boost the CAP antitumoral action on melanoma cells (63). Investigations were also conducted on MCF-7 breast cancer cells. The preliminary findings showed that CAP therapy decreased cellular survivability because apoptosis was accelerated (64). Later, Ninomiya et al. reported how CAP triggered damage to 50% of breast cell lines, irrespective of whether those were the non-invasive MCF-7 or aggressive MB-231 cell lines (65). Eventually, cells isolated from human breast cancer cell metastasis showed the antitumor effect of CAP (65). The colon carcinoma cell lines LoVo, SW480, and HCT-116 have been the subject of additional study. Following CAP administration, these cells reduced survivability, followed by a reduction in cell motility and an increase in B-catenin autophosphorylation (66). The Akt1/Nox2 cascade was later found to be activated by CAP therapy by Ishaq et al. to promote apoptotic in multiple colorectal cells (67). Additionally, the study indicated that just by blocking Srx/Nrf2 signaling, the HT29 cell line’s resistance to ROS-induced cancer mortality might be diminished. Moreover, heterogeneous tumor spheroids challenged to CAP exhibit antitumoral properties that presumably imitate the tumor microenvironment in a dose-dependent fashion. After such CAP treatment, spheroid Ki67 levels decreased, and damage to DNA increased (68). Additionally, CAP use on cervical and uterine cancers has been examined in numerous research, specifically using HeLa cells. During CAP treatment, apoptosis activities are also seen in these cells due to elevated ROS and resultant modifications to the p38 and c-Jun N-terminal kinase (JNK) mechanisms (69). Cell disintegration carried by membrane lipid peroxidation was another reported explanation for this phenomenon. Leukemia cell line applications for CAP tools were also investigated. But, the potential use of CAP in therapeutic settings is yet unknown, so finding the most effective way to use it calls for more research. Furthermore, THP-1 leukemia cell lines were dose-dependently inhibited in-vitro by CAP (70). The application of CAP in immunotherapies is among its most cutting-edge applications. This is feasible since the immune system of humans substantially impacts the development of malignancy and how it progresses. The ability of the immune response to modulate specifically outweighs the power of cancerous cells to suppress immunogenicity (71). Utilization of immunological checkpoint blockades, cell-based treatments, and different cytokines are a few examples of those modifications (72). Certain chemotherapeutic and radiotherapy techniques also cause immunogenic apoptosis (73, 74). Table 1 summarizes the outcomes of CAP treatment on several cancerous cell lines.
Application of CAP for Therapy of Chronic and Acute Wounds
In the late 1990s, the activity of antimicrobial CAP was shown, resulting in its usage in medicine (84). The plasma device was utilized in face rejuvenation operations in the initial clinical study in 2007 (85). Because bacterial infection can significantly impede the healing process, the initial goal of CAP usage in regenerative medicine was to accelerate acute and chronic wound healing by reducing infection (4). Isbary et al. conducted the initial pilot experiments that were randomly assigned (86). These researchers examined the effect of CAP on bacterial invasion attenuation in chronic ulceration wounds and found that it reduced infection significantly without causing any side effects. Shortly afterward, they found that two-minute CAP therapy effectively reduced bacterial burden and enhanced the healing of chronic ulcers (87). Both trials comprised venous, traumatic ulcers, arterial, and diabetic, and decreased bacterial infection was seen irrespective of the kind of bacteria. Venous ulcers, which harm up to 2% of the world's population, are particularly prevalent in chronic leg ulcers (88). Ulcer prevention and treatment are difficult and time-consuming. Table 2 summarizes the CAP uses for chronic wound repair.
Table 2. Applications for chronic wound healing using cold atmospheric plasma (CAP).
|
Type of wound |
Number of Subjects/Patients |
Gas Infused/Plasma Tool |
Exposure time |
Outcome |
Ref(s) |
|
Pressure ulcers in Wistar rats |
--- |
Helium (He)/Plasma jet |
60 seconds/3 x per day/5 days |
Quick collagen synthesis, angiogenesis, and re-epithelialization |
(93) |
|
Pyoderma gangrenosum |
n=2 |
Plasma jet tool with different types of electrodes |
>5 minutes till the entire region was not exposed to radiation/every 2nd day/6-8 times |
Progressive wound repair, absorption, and drying
|
(94) |
|
Chronic venous leg ulceration |
n=14 |
PlasmaDerm® VU-2010 DBD tool |
45 seconds / cm2 (maximum 11 minutes) / 3 x per week / 8 weeks |
Quick decrease in ulcer lesions and potent antibacterial action |
(91) |
|
Chronic leg ulcers |
n=36 |
Argon (Ar)/MicroPlaSter plasma torch |
5 min/day |
Quicker wound healing and reduced microbial burden |
(86) |
|
Chronic pressure ulcers |
n=50 |
Ar/Plasma jet |
1 min/cm2/1 x per week/8 weeks |
Reduced microbial burden, excellent PUSH (Pressure Ulcer Scale for Healing) score |
(92) |
|
Diabetes-related chronic leg ulceration |
n=1 |
Plasma jet tool with different kinds of electrodes |
>5 minutes till the entire region was not exposed to radiation/every 2nd day/3 times |
Progressive wound repair |
(94) |
|
Chronic ulcers |
n=24 |
Ar/MicroPlaSter alpha and beta plasma torch |
2 min/day |
Quicker wound healing and reduced microbial burden |
(96) |
Furthermore, 71% of patients have challenging recoveries, and 15% have ulcers that never healed (89). The complicated nature of treating ulcers in large part stems from the fact that many bacterial strains prevalent there are becoming increasingly resistant to traditional antibacterial therapy (90). Thus, research emphasized mainly the use of cold plasma to treat ulcers. Fourteen individuals participated in a randomized investigation on CAP therapy (91). The remaining patients received concurrent CAP therapy, while the other half received traditional treatment. Medication was given to both categories thrice weekly for 8 weeks, then a four-week surveillance period. Although both groups had fewer arterial ulcers, the CAP group improved more quickly and visibly. A similarly designed trial then examined the impact of CAP on 50 individuals having pressure ulcers (92). The therapies were administered once a week for 8 weeks to the groups of participants receiving conventional treatment and those receiving combination CAP intervention. Following the initial week, the CAP group demonstrated significant improvements and reduced bacterial burden. Wistar rat models have similarly shown CAP benefits regarding pressure ulcer minimization. CAP was applied to experimentally produced ulcers for 60 seconds 3 times for 5 days (93). There was quick collagen synthesis, angiogenesis, re-epithelialization, and enhanced tissue mechanical strength on plasma exposure. Gao et al. looked at the effects of CAP on several chronic wounds, such as pyoderma gangrenosum, enormous genital warts, and ulcers in diabetic feet. An earlier attempt at using antibiotics to treat the patient with pyoderma gangrenosum had failed.
Every two days, for 60-80 minutes, the lesions received a 5-minute dose of radiation. The wounds could fully dry and constrict after 6 cycles of exposure on the third day when exudation was noticeably decreased. Without success, this patient received treatment for an additional six months. After receiving antibiotic therapy, a second patient's pyoderma gangrenosum showed some signs of progress, and they were then given this CAP treatment. With eight CAP administrations, the lesion entirely vanished. Four months later, there was still no sign of a recurrence. A person with a diabetic foot ulcer for 2 months also had cured ulceration following 4 different CAP therapies (94). Ultimately, two separate studies explored the feasibility of simultaneously treating chronic leg ulcers with octenidine disinfectant and CAP devices. The authors believed combining this disinfection with cold plasma should produce better outcomes than either method applied alone (95).
The main goal of using CAP is to promote a contemporary method of wound cleaning. Moreover, CAP therapy has proven effective in wound repair attributed to the activation of monocyte, improved cutaneous microcirculation, the multiplication of fibroblasts and keratinocytes, and cellular motility (97). The fibroblasts and keratinocytes are very significant in the final stages of wound healing (98). Upon cell lines, an in-vitro analysis has shown that CAP treatment benefits keratinocyte and fibroblast proliferation (99). Despite a brief exposure to CAP, the keratinocyte and fibroblast cell lines HAcaT and MRC5 displayed enhanced motility. In addition to alterations in adherent junctions and cytoskeletal mobility, this involves the down-regulation of E-cadherin and integrins and reduced gap-junction protein function at the cellular stage. In that work, the researchers analyzed the in-vivo plasma activity in mice models. They revealed that the detected wound repair is attributable to the generation and activity of UV exposure, RNS, ROS, and NO (99). The CAP action on keratinocyte function was also validated by numerous researches. After exposure to CAP, these cells concurrently generated more b1-integrin and less EGFR and E-cadherin (100). Following CAP administration, keratinocytes exhibited much higher amounts of ROS, which caused numerous cell adaption processes (101). Approximately 260 genes with variable expressions, such as an antioxidant enzyme, growth regulator, and cytokine. There was a variable expression of more than 260 genes, among those that code for cytokines, growth hormones, and antioxidants. HSP-27, a heat shock protein that protects cells and helps control cell growth and maturation, was also substantially upregulated (101). According to Schmidt et al., the protein p53 pathway could serve as a key node for connections between cold plasma and cells in keratinocytes. Furthermore, the researchers hypothesize that the mitogen-activated protein kinase cascade should alter the p53 responses since the ATR and ATM oxidative detectors are more effective (102). A new analysis revealed remarkable "cross-talk" among keratinocytes and fibroblasts impacted by CAP (103). These cells were co-cultured, and the findings revealed that plasma administration enhanced Salvador-Warts-Hippo signaling function and markedly increased the transcriptional activity of its cofactor, i.e., Yes-associated protein (YAP). In addition, only fibroblasts showed higher expression of the YAP targeting markers CYR61 and CTGF, which are downstream effectors of this system. However, the administration of antioxidants could reduce this enhanced expression. The most significant finding was that CAP-treated fibroblast-conditioned media was required for improved HaCat keratinocyte cell motility. The researchers concluded by assuming that paracrine activation of keratinocytes occurs due to fibroblasts secreting Cyr61 and CTGF. This is supported by the fact that, even in the absence of CAP-treated fibroblasts, the HaCat lines moved more freely after exposure to recombinant Cyr61 and CTGF (104). Intriguingly, 2nd and 3rd-degree burn wound healing was expedited by cold plasma in animal mice models. The main reason for this was enhanced angiogenesis.
The expression of CD31 and PDGFRβ, two pro-angiogenic factors, enhanced, and NO production was expedited at the cellular level. A rise in TGFβ1 function and activation of VEGFR2/VEGFA signaling was also observed (105, 106). The beneficial effects of CAP were also demonstrated in the recovery of chemical injuries in rats after being exposed to sulfuric acid. Here, a daily 40-second contact with CAP speeds up the healing process. After 21 days, the CAP-treated wounds nearly vanished, while the not treated wounds were still distinctly visible. The authors also mentioned that wounds treated with CAP had a different metabolic profile. The subsequent oxidative stress markers showed altered levels, which supported this. Compared to normal controls, those receiving plasma-modified polyurethane wound wrapping, and those in the group receiving traditional wound healing, the malondialdehyde concentrations were higher, and the catalase, glutathione peroxidase, and reduced glutathione concentrations were reduced in the CAP group. During the 21-day therapy, these groups had differential white blood cell kinetics, fibrinogen synthesis, and C3 levels (Complement component 3). White cell numbers were typically the least in the CAP group and greatest in the traditional wound repair group. The levels of C3 and fibrinogen were both higher in the groups receiving traditional wound repair and were lower in the groups receiving CAP therapy (107). Then, Betancourt-Angeles et al. showed that cold plasma hastened the recovery of human burns (108). Following a 3-minute CAP exposure, irritation and pain were minimized, and subsequent 3-min repeat exposure after 16 hours resulted in markedly faster recovery and the development of newer tissue. Since this was a clinical study analysis, the researchers didn't explore the molecular processes before, during, or after plasma treatment. Furthermore, earlier studies have shown evidence that angiogenesis and growth factor stimulation have occurred. Table 3 summarizes the CAP uses for acute wound repair.
Table 3. Applications for acute wound healing using cold atmospheric plasma (CAP).
|
Type of wound |
Number of Subjects / Patients |
Gas Infused / Plasma Tool |
Exposure time |
Outcome |
Ref(s) |
|
Traumatic wound |
n=2 |
Plasma jet tool with different types of electrodes |
20 min for the whole wound / every 2nd day |
Reducing wound inflammation and discontinuing wound care after three healing treatments |
(94) |
|
CO2 laser skin damage |
n=20 |
Ar/kINPen® MED plasma jet |
3-10 seconds/3 days |
No evidence of plasma adverse effects enhanced wound repair |
(113) |
|
Dog bite wound |
--- |
Ar/kINPen® VET plasma jet |
< 2 minutes of treatment under in-vitro settings |
Possibility of antibacterial action on bacterial strains often found in dog bite wounds and dog saliva |
(110) |
|
Wound after genital wart |
n=1 |
Plasma jet tool with different kinds of electrodes |
>5 minutes till the entire region was not exposed to radiation/every 2nd day/2 times |
Progressive wound repair |
(94) |
|
Burn wound |
n=1 |
He/Plasma jet |
3 min/2 times with 16 hour between 1st and 2nd uses |
Reduce urticarial and pain following 1st use and re-epithelization following 2nd use |
(108) |
|
Fractional CO2 laser skin damage |
n=12 |
Ar/kINPen® MED plasma jet |
--- |
Comparable to the results of normal therapy, but with decreased skin inflammation and average smoothness |
(112) |
Researchers have also explored how CAP affects other acute wounds. Patients in one research had skin transplants on their lower extremities of various sizes. Two groups of patients were developed, one of which received a dummy while the second received CAP therapy. The overall findings revealed that the patients in the CAP group had a significantly improved recovery path on the 2nd day following CAP treatment (109). Another intriguing study explored the possibility of applying plasma to dog bite injuries (110). In this study, the researchers examined the effects of CAP on the bacterial species Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Pasteurella multocida, Streptococcus canis, and Staphylococcus pseudintermedius which are frequently detected in dog saliva. The majority of these bacteria were strongly inhibited from proliferating in-vitro by CAP. But depending on the growth stage of bacteria and the duration of the treatment, certain differences between the species were found. In a relevant experiment conducted by the researcher, it was reported that CAP had lesser total disinfection effectiveness than saline lavages and polyhexanide-biguanide (111). Although there were differences, they were not statistically relevant, and the disinfectant used did not affect how quickly the wounds healed. Thus, additional research would be helpful to draw firm findings about the utility of CAP in managing dog bite wounds. Thus, it is reasonable to anticipate that the existing capacity for CAP administration in such acute wounds will expand, particularly as the rising resistance of these bacterial strains makes treatment more challenging (111). In a different research, normal subjects had elective laser-ablative skin infections. The researchers subsequently applied CAP to their damaged wounds, and their three-day scheduled regimen at 30-second periods was adequate to enhance wound repair (106). Nishijima et al. investigated cold plasma's potential to expedite recovery in mild epidermal injuries caused by a fractional CO2 laser. External ointment administration, such as basic fibroblast growth-factor spraying, petrolatum, steroids, and fullerene-containing gels, is the recommended therapeutic therapy for these wounds. The trial subjects were separated into 4 groups: (1) without therapy; (2) CAP therapy; (3) application of betamethasone valerate-containing ointments; and (4) application of basic fibroblast growth factor spraying. Despite no substantial variations in the group's total healing process, the CAP group had the least skin inflammation and average smoothness (112).
Application of CAP to Trigger Proliferation in Progenitor and Stem Cells
The replication of progenitor cells may also be modulated by CAP, which might lead to their usage in restorative therapy and biomedical nanotechnology. Indeed, limited investigations have explicitly explored this concept, according to a research analysis by Park et al. CAP tools could encourage progenitor cells originating from adipose tissue to proliferate without altering their intrinsic characteristics (114). As soon as 72 hours following CAP therapy, the same researchers observed that progenitor cell multiplication was 1.57 times greater. The research also documented the effect of NO triggered by CAP (114). The introduction of NO significantly reduced the CAP device's favorable enhancement in progenitor cell multiplication. Adipose tissue cells that had not been subjected to CAP also increased at a level greater than that of reference cells but considerably fewer than that of CAP-affected cells when administered with the NO donor DETA-NONOate (diethylamine nonoate). Based on these findings, higher NO content is a significant component of enhanced multiplication, although it is probably not unique (114). In osteoprogenitor MC3T3-E1 cells after CAP exposure, zero cytotoxicity or other adverse effects have been reported.
Additionally, CAP increased concentrations of accumulated NO. This NO was delivered to the cellular components, and modulating its concentration in developed cells was possible. Early osteogenic development was therefore triggered as a response, which was accomplished irrespective of the condition of the pro-osteogenic growth factor under these situations (115). Another investigation on the influence of CAP on osteo-differentiation focused on the effects on MC3T3-E1 murine osteoblast cell lines (116). CAP's osteogenic differentiating modulatory impact was equivalent to that examined in the osteogenic differentiating environment. The researchers also reported important alterations in the mechanistic pathways after the CAP treatment. The osteogenic markers ALP, COL-1, OCN, and RUNX2 were expressed more frequently with a decreased MAPK and PI3K/AKT signaling.
Moreover, CAP impacted FOXO1's autophosphorylation, which is essential for osteoblast multiplication and oxidative equilibrium and a key regulator of bone growth (117). Additionally, the function of CAP on adult embryonic stem cells (hPDL-ESCs) derived from periodontal tissues was explored. Once more, there was no cytotoxicity or an adverse response. Interestingly, this CAP therapy restricted hPDL-ESC recruitment without affecting total cellular survivability and triggered dissociation (118). Similarly, introducing CAP tools can stimulate the advancement of bone marrow and hematopoietic-derived stem cells. Comparing these cell lines to normal cells, the multiplication increased in both (42). The researchers also revealed that the upregulation of prominent stem cell biomarkers CD105 and CD44 was approximately 5-fold more significant in bone marrow cell cultures. NANOG, SOX2, and OCT4 proteins were amplified more frequently in both cell types. Therefore, it may be hypothesized that CAP administration can affect this cell division stage because the transcription of genes that regulate the G1-S cell division shift is also enhanced (42). Treatment for the catastrophic central nervous system (CNS) and neurological condition impairment is still challenging.
Moreover, neural stem cells (NSC) may enhance how they are treated, and multiple researchers have explored how CAP stimulates NSC multiplication. They demonstrated that CAP therapy greatly accelerated the multiplication and maturation of C17.2 (mouse neural progenitor cell line) (119). However, following treatment to CAP, approximately 75% of NSC transformed into neural cell lines, a proportion higher than that attained by particular growth stimuli. Elevated concentrations of tubulin III (microtubule protein) activity are considered a characteristic hallmark for this kind of nerve cell separated from NSCs following CAP therapy (120). Current work, although, reveals that significant tubulin III activity is also prevalent in various progenitor types of cells (121). Additionally, comparatively limited oligodendrocyte development from NSC was reported by the researchers of this experiment, and activation of the characteristic O4 protein signature was relatively marginally greater (121). Table 4 summarizes the outcomes of CAP therapy on progenitor and stem cells throughout in-vitro settings.
Application of CAP for Dental Medicine
The mainstay of conventional cleaning techniques and oral cavity disinfection is the utilization of laser devices, mechanical infection removal, or antimicrobial solutions. However, the first two techniques risk damaging tissues mechanically or thermally. With CAP devices, this risk can be considerably reduced (123). Another benefit of CAP is that it is quite simple to apply its discharge to difficult-to-reach oral cavity regions and uneven surfaces. Applying the discharge from sufficiently tiny devices directly to the tooth canal (124). Finally, CAP can be applied to specific locations in the oral cavity, unlike liquid antimicrobial solutions, and there are no negative results from microbial liquid use after CAP therapy. The resistance of the bacterial strains found frequently in tooth biofilm is rising. Endocarditis, necrotizing pneumonia, and other systemic conditions can develop due to neglecting to remove tooth biofilm (125). Delben et al. reported that the CAP application's antibacterial effects effectively attenuated Staphylococcus aureus and Candida albicans, which are frequently identified in dental plaque (126).
Table 4. The outcomes of CAP therapy on progenitor and stem cells in in-vitro settings are outlined below.
|
Cell Types |
Gas Infused/Plasma Tool |
Exposure time |
Outcome |
Ref(s) |
|
Adipose-derived stromal cells |
Helium (He)/Dielectric barrier discharge (DBD) |
3 minutes/ specimen (1 hour of plasma-activated medium culture) |
Cell growth is halted, alteration in morphological characteristics with variations in p16 expression, cellular phenotype during aging, an elevation in p53/p21 impairment |
(122) |
|
Adult embryonic stem cells derived from periodontal tissues |
He/Plasma needle |
10-120 seconds |
Decreased cell motility, absence of stickiness |
(118) |
|
Stem cells isolated from adipose tissues |
He/DBD |
50 seconds per hour/10 times |
2 times increased stem cell growth in-vitro following CAP therapy, increased NF-κB, ERK1/2, and Akt cascade expression, elevated concentration of nitric oxide |
(114) |
|
Mouse neuroblastoma stem cell (N2a) |
(Nitrogen) N2+ (Oxygen) O2/DBD |
1-10 minutes |
Cell multiplication increases following CAP treatment, stimulation of ERK/Ras/Trk cascade, cytochrome c oxidase was hindered by an elevation in NO |
(38) |
|
Osteoprogenitor cells (MC3T3-E1) |
Non-thermal biocompatible plama (NBP)-DBD |
1-10 minutes |
FOXO1 transcription factor dephosphorylation, upregulated p38 activation, MAPK, and Akt/PIK3 activation are reduced |
(116) |
|
Mouse neural stem cells (C17.2- NSC) |
O2/Plasma jet He |
60 seconds |
Increased cellular levels of multiplication and development |
(119) |
|
Osteoprogenitor cells (MC3T3-E1) |
DBD discharge NO-plasma nozzle setup |
30-180 seconds |
The potential of NO infiltrating intracellular membrane and an elevation in NO in the reference medium, absence of cellular cytotoxicity |
(115) |
Additionally, the microbial burden decrease achieved with the CAP tool was equivalent to that achieved with fluconazole or benzylpenicillin treatment. The antibacterial activity of cold plasma on various microbes in dental lesions, mainly notably Streptococcus mutans, was validated by research with an identical objective (126). Additionally, the CAP in-vitro antiproliferative activity on established cell lines was higher than conventional chlorhexidine disinfectant usage. The use of CAP tools to lessen the microbial burden in the tooth canal has also been explored in multiple experiments. Thus, ex-vivo CAP treatment for 5 minutes indicated the clearance of the bacterial biofilm to a thickness of 1 mm through the technique of scanning electron microscope (SEM) (127). Then, using 100 specimens of removed and sanitized teeth, Armand et al. mimicked an infestation with Enterococcus faecalis. In infected dental canals, this bacterium is relatively common (128). SEM revealed that O2/He plasma was the most efficient in lowering the microbial burden, and He plasma showed comparable efficiency as photosensitizing treatment.
Moreover, the researchers observed that the tooth canals' morphology significantly affected the outcome. In the straight canals, the damage was greater. The rewards of investigating the potential and restrictions of applying CAP to the dental region were then reported by Shahmohammadi Beni et al. (129). There, researchers examined the use of CAP concerning the surface features of the oral region, focusing particularly on the scattering of OH radicals. According to Dong et al., plasma enhances the surface dentine's potential to adhere to other dental tissues (130).
Additionally, using CAP is intended to improve the connections between hybrid inner material and fiber-reinforced posts (131). Titanium components in the dental cavity are also strongly influenced by cold plasma. Therefore, CAP must improve the titanium coating’s ruggedness and hydrophilicity (132). These two characteristics can enhance osteoblast development, cell attachment, and multiplication, and these three characteristics can speed up osteointegration (133). However, the ruggedness produced by CAP treatment might lead to larger bacterial development, and CAP's antibacterial properties can mitigate this adverse impact. CAP tools are ideally suited for managing peri-implantitis due to these properties. CAP also alters zirconium formations. Following CAP therapy, Yang et al. observed a reduction in microbial burden, an enhancement in hydrophilicity, and no alteration in the morphology of the zirconium formation (134). Table 5 summarizes the outcomes of CAP therapy on oral and dental region components both in-vitro and ex-vivo.
Table 5. Outlines the results of exposing CAP to oral and dental region cavity components in in-vitro and ex-vivo settings.
|
Gas Infused/Plasma Tool |
Exposure Time |
Rational of CAP therapy |
Outcome |
Ref(s) |
|
O2/Argon (Ar)/Plasma jet tool |
5 min/one extracted tooth |
Tooth canal disinfection |
Bacterial infection in the tooth canal has been effectively reduced ex-vivo to a thickness of 1 mm. |
(135) |
|
Plasma jet tool; Ar/Hollow dielectric barrier discharge (HDBD) tool; Ar/Plasma brush; He+N2, O2, N2, and Ar/low-pressure plasma tool |
30-90 seconds/specimen; 2-6 minutes/specimen; 30 seconds/specimen; 10 minutes/specimen |
Optimization of dental structures |
Improvement of connections between the hybrid inner material and fiber-reinforced posts; a rise in the hydrophilicity of zirconium and titanium formations; improved adherence to dentin |
|
|
Ar/kINPEN®MED plasma jet |
60 seconds/specimen |
Dental biofilm reduction |
Antimicrobial activity against Staphylococcus aureus and Candida albicans; regenerated oral epithelium had no adverse effects |
(126) |
|
O2/He; He/Plasma jet tool |
2-8 minutes/specimen |
Tooth canal disinfection |
Remarkable decrease in ex-vivo infection with Enterococcus faecalis; improved effectiveness when using O2/He as a carrier gas |
(127) |
CAP Devices for Medical Applications
The argon-driven microwave plasma torch SteriPlas (ADTEC, Hunstlow, UK), the DBD-based PlasmaDermVR (CINOGY GmbH, Duderstadt, Germany), and plasma careVR (terraplasma medical GmbH, Garching, Germany), and the argon-driven HF plasma jet kINPen®MED (Neoplas tools GmbH, Greifswald, Germany), all of which using atmospheric oxygen as carrier gas. Their primary function is to speed up the recovery of prolonged wounds and other epidermal conditions caused by pathogens (136). This is important to highlight since there are a variety of distinct products on the market that are promoted as being advantageous for "plasma medicine," but they lack or have insufficient physiological, technological, biological, or medical evidence to support this. Therefore, it will be important to develop a significantly superior systematization and standardization of plasma technologies available for exploitation in the healthcare sector in the upcoming years. Over importantly, a stronger distinction between CAP devices and other plasma-based therapeutic tools is required. To achieve improved organization, consider the following criteria:
- CAP produces plasma with a temperature lower than 40ᵒ C nearby or in close interface with the object to be cured (a skin or wound).
- Tools that produce working gases or gas combinations from plasma, such as O3 and NO (137, 138).
- Tools for cutting, tissue burning, and blood coagulation that use electrosurgical plasma and predominantly use thermal effect (139).
The lack of a variable or combination of variables to regulate and evaluate plasma efficiency that may be exploited as a "dosage" in the same manner as laser treatment, radiation therapy, and phototherapy is one of the major technical limitations of using plasma tools. Based on the current understanding, biomedical CAP impacts are the outcome of intricate connections between plasma constituents and the morphology and constituents of healthy cells, with a dominant involvement for RNS and ROS accompanied by (electrical fields and UV radiation (97). A single component or plasma parameter has not been established that can be directly associated with particular biological impacts or treatment results. Therefore, treatment duration and energy input are often used to regulate plasma's influence on biological research and therapeutic applications. The main problem in the preliminary investigation of plasma therapy may be the discovery and specification of such a variable or combination of variables for device-independent regulation of physiological plasma overall effectiveness (140). A sample screen for the fundamental assessment of CAP efficiency may be implemented as a platform for an initial but workable comparison of various CAP tools in the interval. A first recommendation for such screening is established with the German DIN SPEC 91315, "Basic standards for plasma supplies in healthcare” (141). The following physical assessment parameters are postulated: thermal activity, irradiation measurements in the 200-900 nm band, gas discharges, gas/plasma temperature, current movements, and optical emission wavelength. In-vitro studies to establish if certain microbes have been rendered inactive and in-vitro studies to assess the survivability of cultured eukaryotic cells are featured in the biological assessment parameters.
Moreover, it is suggested that the content and quantity of RNS and ROS produced by the plasma tool be approximately estimated using the identification of chemical entities produced by CAP therapy of aqueous solution. The integration of physical, biological, and chemical testing suggested by DIN SPEC 91315 is an initial approach toward a more comprehensive standardization of plasma supplies for use in therapy. This testing screen rates their efficiency and provides relevant data on human safety. Developing an engineering target comparable to the human body is an alternative strategy to enhance plasma identification in-vitro. This target may be used to detect connections between plasma and the target and how those connections influence the properties of the plasma (142). Biorelevant marker molecules viz hemoglobin or cysteine can be employed to characterize the pharmacological activity of CAP in more depth and determine its biomedical effectiveness (143). This research aims to describe CAP tools as extensively and effectively as feasible to assess their predicted pharmacological outcomes in terms of both treatment applicability and toxicity (144). Adapting CAP devices to the demands of individual medical applications is also a continuing issue in CAP device design and development. Currently, the focus of medically-approved CAP devices is on external applications (skin inflammation, wounds). It is necessary to recognize greater surface regions, particularly in wound recovery. It is possible to create DBD electrode configurations that are flat and planar, allowing them to span the whole surface area. The PlasmaDerm Dress system is one such product now on the market (CINOGY GmbH, Duderstadt, Germany). Plasma jet arrays may cure greater areas as well. Automated devices may help in the process of moving spot-like plasma jets over the area being treated (145).
As plasma technology becomes more widely used in the medical field, there will likely be a growing need to tailor plasma devices to meet the unique requirements of various medical settings. Precision control over treatment factors like distance or temperature might make comparable CAP devices suitable for use on body surfaces in ophthalmology. There is a need for individualized plasma devices to provide safe and comfortable plasma therapy of the oral cavity as part of the CAP's potential use in oral medicine. Miniaturized catheter-shaped plasma devices are being developed for endoscopic and other minimally invasive surgical procedures (146). The biggest obstacle for these uses is ensuring steady and efficient plasma formation in tiny and lengthy body cavities that are moist and poorly ventilated. Perhaps the same holds for plasma devices used during laparoscopy (147). Another need for all these intra-body plasma applications is developing a reliable navigation system. When developing and refining plasma tools for particular therapeutic purposes, it is essential to consider the sophistication of plasma production, surveillance, and regulation on the one side and the unique implementation requirements and conditions in healthcare on the other. The investigation and advancement of CAP tools is a distinctive and essential domain in plasma medicine due to the demand that most CAP tools’ essential characteristics be recognized and ideally optimized before initial in-vivo experiments (Figure 4).
Safety Evaluation of the CAP Devices
Every plasma system, either DBD, APPJ, or CDT, generates a "combination of chemicals" of various quantities of electrical charges, reactive oxygen (O3) and nitrosative (NO and NO2) species, electrons, UV radiation, and ions. The system exploited, the mode of transmission, the inert gas, the parameters of the plasma, the duration of administration, and the environmental factors all influence the plasma components and their quantities, as well as the current across the targets. It is commonly documented that many reactive oxygen-nitrogen species (RONS) are cytotoxic for individuals if they surpass a specific frequency, strong electrical currents across the skin can induce blistering or inflammation, and UV radiation (mainly short-wavelength, i.e., 200-280 nm) causes DNA alterations that cause mutations and apoptosis. ROS released by N2 and air plasma discharges from a spray-nozzle device has also been reported to trigger mitochondria-induced apoptosis, which acts as a proapoptotic signal (148). This is a significant discovery for prospective use in cancer treatment, but it could have negative effects on other applications, including the treatment of wounds. Applications in-vivo have explicitly considered the oxidative stress induced in many cell lines, including keratinocytes and myofibroblasts (149). Hence, a detailed safety evaluation must be performed before human clinical studies to validate that the application of plasma on people is "suitable" and therefore establish an optimal "safe" therapeutic index for the intended use (150). For this evaluation, comprehensive plasma testing must be conducted to pinpoint plasma constituents and calculate their corresponding values or quantities for various plasma configurations and treatment durations, utilizing multiple exploratory and hypothetical computational methods. The outcomes of these assessments should then be matched to previously reported threshold limits and interpreted appropriately.
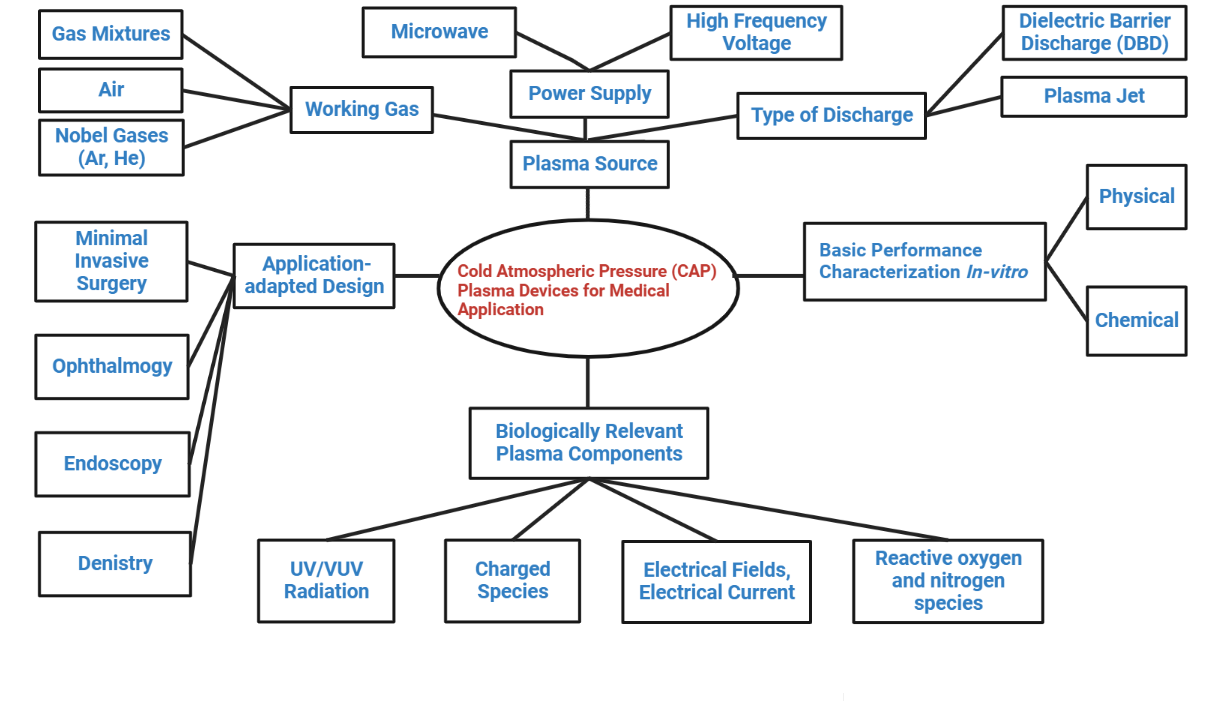
Figure 4. The foremost crucial factors should be considered before in-vivo usage whenever CAP tools for clinical uses are designed and developed for particular purposes.
UV emission
The stratum corneum safeguards against short wavelength; hence the maximal permissible UV dosage for good, undamaged skin surface, which the International Commission proposes on Non-Ionizing Radiation Protection (ICNIRP), is 3 mJ/cm2. Furthermore, most in-vivo plasma therapies are used on skin injuries, i.e., prolonged wounds. Presently, there are only guidelines in a European Commission Report (SCCP report 0949/05) for exposed skin; regrettably, there are no restrictions for maximal UV exposures in injured skin (151). Indirect argon plasma tools (MicroPlaSter alpha and beta), the only plasma tools used in a clinical study in individuals with prolonged inflamed scars, also emit UV light. The UV dosages generated were under the ranges specified in the standards and regulations. Still, researchers and medical professionals also decreased the plasma treatment duration from 5 to 2 minutes to provide patients with a broader safety tolerance, particularly to minimize potential long-term repercussions (86). For therapy periods of up to 1 minute, the UV dosages for the combination plasma tools viz MiniFlatPlaSter, FlatPlaSter, and HandPlaSter were reported to be 100 folds smaller than the official ICNIRP standard (152). The ideal intervention course is designing plasma tools with as few short wavelengths as feasible. As a result, there is a demand for updated standards and proposals for emerging innovations.
Toxic gas emission
In CAPs, reactive oxygen (O3) and nitrogen (NO and NO2) species are synthesized by electron fragmentation interactions with ambient oxygen and nitrogen, resulting in more than 600 biochemical events. Since not all RONS are subject to limitations, this investigation emphasizes categories for which there are regulations and which are the most effective components of plasma (153).
O3
The National Institute for Occupational Safety and Health (DC, USA) issued a recommended dosage range for O3 of 0.1 ppm for 8 hours of sustained breathing. The Occupational Safety and Health Administration also issued a 15-minute O3 exposure threshold of 0.3 ppm. Plasma tools that generate O3 concentrations significantly lower these reported threshold levels, such as the Kin Pen MED and MicroPlaSter alpha and beta systems, which have previously been used in clinical studies on people, are safe to use. However, air plasmas, i.e., MiniFlatPlaSter, FlatPlaSter, and HandPlaSter, create greater O3 values and cannot be utilized for primary breathing. Therefore, it is plausible to create plasma molecular patterns that prioritize nitrogen chemistry instead of oxygen, resulting in quenching that lowers the production of O3 (154).
NO and NO2
The maximum levels of NO and NO2 recommended by the National Institute for Occupational Safety and Health for 8 hours of sustained breathing are 25 ppm and 5 ppm, accordingly. At the working range of 20 mm for the MicroPlaSter systems, the NO2 values are less than 1 ppm (155). NO values under 25 ppm for the MiniFlatPlaSter were reported for therapy periods up to a few minutes. Additionally, it was revealed that the discharge of NO2 is based on specific plasma factors (power source and therapy duration) and may thus be adjusted as necessary (154).
Target Exposed to an Electrical Current
CAPs include ions and electrons within their contents. Consequently, when implemented in-vivo in clinical studies, electrical currents may penetrate across the target, i.e., the skin. The threshold levels for normal undamaged human skin documented by ICNIRP range from 0.5 mA at 1 kHz-20 mA at 100 kHz. The Canadian Health Authority dropped these levels to 100 µA in 2008 for several factors. Moreover, the International Standard IEC 60601-1 for healthcare electrical devices applies to this 100 µA level. The currents through the target can be ignored in plasma torches or jets (MicroPlaSter alpha and beta). However, a tiny percentage of charged ions may penetrate the skin. The ICNIRP current restrictions are not surpassed since most DBD devices function more frequently. Therefore, precise measurement and surveillance of currents produced by DBD plasmas are required to provide "safe" application to human skin (156).
Conclusion
Plasma medicine is an interdisciplinary domain of research that combines plasma physics, medicine, biology, plasma chemistry, and engineering. It originated from an analysis of the use of atmospheric plasmas at low-temperature (or cold) conditions in biomedicine. Science and medicine have advanced due to adopting the CAP plasma technique in the life sciences sector. It has recently advanced scientifically to great levels. It has been effectively used for various functions, including disinfection, cancer suppression, wound healing, and the deactivation of SARS-CoV-2. For the management of persistent and infectious lesions, three CAP systems have currently received clinical use certification. Even though there are still many unanswered questions surrounding the process of the interaction of plasma with biological matter, several fundamental concepts are understood.
The biological and therapeutically advantageous plasma effects are caused by reactive oxygen species (ROS) and reactive nitrogen species (RNS) produced by the plasma. Depending on the biological synthesis of RNS and ROS in reactions to plasma emissions, the present review described several aspects of plasma therapy in neuroscience, particularly in anti-glioblastoma, neuro-differentiation, and neuroprotection. Though CAP plasma has attained the stature of standard medical care in several areas, such as disinfection, sterilization, wound therapy, etc., mainly primary and pre-clinical reports are obtainable for its efficacy in other sectors. To maximize the CAP plasma technology's therapeutic effects, more advancements and expansions would be possible with a profound knowledge of the principle behind its effectiveness. Novel aspirations will likely arise from producing novel plasma devices and altering previously existing types. To efficiently compare the results of various observations, this scenario must standardize the techniques to describe the plasma devices internationally. It is impossible to ignore the possibility that CAP plasma administration has some minimally negative molecular effects. Research is still being carried out on various data and statistics, but preliminary studies suggest that the many merits of CAP plasma surpass any potential challenges. We all anticipate that CAP plasma will soon become part of clinical practice. In brief, an interdisciplinary research platform is developing with professionals from numerous disciplines, such as biochemistry, plasma physics, medicine, molecular biology, etc., to solve problems coordinatedly. However, recent studies on the benefits of cold plasma continue to use solely animal models and cell lines. The expanded evaluation of CAP's impact on cancer and regenerative medicine investigations in human individuals is crucial. It should prove advantageous, although these tests are more available and simpler. Chemotherapy and regenerative medicine treatments may become much less expensive if CAP is eventually included in standard medical care. The most crucial benefit of CAP therapy is that it will be less intrusive and less stressful for patients. Finding important plasma-generated ROS/RNS and monitoring their impact on various neurons, including tumor tissues, will become increasingly crucial in future investigations.
Further research will determine how these species originated, how they are transported to and inside cells, what interactions are implicated in these activities, and how their impacts spread to other cells. The existing state of research is sufficient to demonstrate that plasmas can (ultimately) be used in the CNS specifically for specific molecular transportation of pharmaceuticals. Plasmas may eventually be able to develop certain species that spread into the tissue cells and result in the intended biochemical and medicinal consequences by manipulating the variables.
Declarations
Acknowledgment
The authors would like to acknowledge Girijananda Chowdhury University, Guwahati, Assam.
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Braný D, Dvorská D, Halašová E, Škovierová H. Cold atmospheric plasma: A powerful tool for modern medicine. International journal of molecular sciences. 2020;21(8):2932.
- Gangemi S, Petrarca C, Tonacci A, Di Gioacchino M, Musolino C, Allegra A. Cold Atmospheric Plasma Targeting Hematological Malignancies: Potentials and Problems of Clinical Translation. Antioxidants. 2022;11(8):1592.
- Sakudo A, Yagyu Y, Onodera T. Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. International journal of molecular sciences. 2019;20(20):5216.
- Bernhardt T, Semmler ML, Schäfer M, Bekeschus S, Emmert S, Boeckmann L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxidative medicine and cellular longevity. 2019;2019.
- Foster JE, Kovach YE, Lai J, Garcia MC. Self-organization in 1 atm DC glows with liquid anodes: Current understanding and potential applications. Plasma Sources Science and Technology. 2020;29(3):034004.
- Johnson MJ, Boris DR, Petrova TB, Walton SG. Spatio-temporal characterization of a pulsed DC atmospheric pressure plasma jet interacting with substrates. Journal of Physics D: Applied Physics. 2020;54(8):085202.
- Al Qaseer SM, Khalaf MK, Salih SI, editors. Optimal Power of Atmospheric Pressure Plasma Jet with a Simple DBD Configuration for Biological Application. Journal of Physics: Conference Series; 2021: IOP Publishing.
- Fiebrandt M, Lackmann JW, Stapelmann K. From patent to product? 50 years of low‐pressure plasma sterilization. Plasma Processes and Polymers. 2018;15(12):1800139.
- Pina-Perez M, Martinet D, Palacios-Gorba C, Ellert C, Beyrer M. Low-energy short-term cold atmospheric plasma: controlling the inactivation efficacy of bacterial spores in powders. Food Research International. 2020;130:108921.
- Domonkos M, Tichá P, Trejbal J, Demo P. Applications of cold atmospheric pressure plasma technology in medicine, agriculture and food industry. Applied Sciences. 2021;11(11):4809.
- Wang Z, Xu S, Liu D, Wang C, Chen J, Zhang J, et al. An integrated device for preparation of plasma‐activated media with bactericidal properties: An in vitro and in vivo study. Contributions to Plasma Physics. 2022;62(3):e202100125.
- Janiszewska D, Szultka-Młyńska M, Pomastowski P, Buszewski B. “Omic” Approaches to Bacteria and Antibiotic Resistance Identification. International Journal of Molecular Sciences. 2022;23(17):9601.
- Hoffmann C, Berganza C, Zhang J. Cold Atmospheric Plasma: methods of production and application in dentistry and oncology. Medical gas research. 2013;3(1):1-15.
- Elaissi S, Charrada K. Simulation of Cold Atmospheric Plasma Generated by Floating-Electrode Dielectric Barrier Pulsed Discharge Used for the Cancer Cell Necrosis. Coatings. 2021;11(11):1405.
- Fridman G, Peddinghaus M, Balasubramanian M, Ayan H, Fridman A, Gutsol A, et al. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry and plasma processing. 2006;26(4):425-42.
- Laroussi M. A brief note on the history of the dielectric barrier discharge and its application for biological decontamination. IEEE Transactions on Radiation and Plasma Medical Sciences. 2021;6(1):121-5.
- Rueda V, Diez R, Bente N, Piquet H. Combined Image Processing and Equivalent Circuit Approach for the Diagnostic of Atmospheric Pressure DBD. Applied Sciences. 2022;12(16):8009.
- Matsusaka S. Control of particle charge by atmospheric pressure plasma jet (APPJ): A review. Advanced Powder Technology. 2019;30(12):2851-8.
- Lata S, Chakravorty S, Mitra T, Pradhan PK, Mohanty S, Patel P, et al. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Materials Today Bio. 2021:100200.
- Korzec D, Andres T, Brandes E, Nettesheim S. Visualization of activated area on polymers for evaluation of atmospheric pressure plasma jets. Polymers. 2021;13(16):2711.
- Lu X, Laroussi M. Dynamics of an atmospheric pressure plasma plume generated by submicrosecond voltage pulses. Journal of applied physics. 2006;100(6):063302.
- Teschke M, Kedzierski J, Finantu-Dinu E, Korzec D, Engemann J. High-speed photographs of a dielectric barrier atmospheric pressure plasma jet. IEEE Transactions on Plasma Science. 2005;33(2):310-1.
- Lu X, Naidis GV, Laroussi M, Reuter S, Graves DB, Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Physics Reports. 2016;630:1-84.
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. nature. 2020;579(7798):270-3.
- Dehning J, Zierenberg J, Spitzner FP, Wibral M, Neto JP, Wilczek M, et al. Inferring change points in the spread of COVID-19 reveals the effectiveness of interventions. Science. 2020;369(6500):eabb9789.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New England journal of medicine. 2020;382(12):1177-9.
- Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England journal of medicine. 2020;382(16):1564-7.
- Wang B, Wu H, Wan X-F. Transport and fate of human expiratory droplets—A modeling approach. Physics of Fluids. 2020;32(8):083307.
- Garcia G, Sharma A, Ramaiah A, Sen C, Kohn D, Gomperts B, et al. Antiviral drug screen of kinase inhibitors identifies cellular signaling pathways critical for SARS-CoV-2 replication. BioRxiv. 2020.
- Bhardwaj R, Agrawal A. Tailoring surface wettability to reduce chances of infection of COVID-19 by a respiratory droplet and to improve the effectiveness of personal protection equipment. Physics of Fluids. 2020;32(8):081702.
- Chen Z. Development of new cold atmospheric plasma devices and approaches for cancer treatment: The George Washington University; 2018.
- Li W, Yu H, Ding D, Chen Z, Wang Y, Wang S, et al. Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapy. Free Radical Biology and Medicine. 2019;130:71-81.
- Babaeva NY, Naidis GV. Modeling of plasmas for biomedicine. Trends in biotechnology. 2018;36(6):603-14.
- Chan K, Sridhar S, Zhang R, Chu H, Fung A-F, Chan G, et al. Factors affecting stability and infectivity of SARS-CoV-2. Journal of Hospital Infection. 2020;106(2):226-31.
- Chen Z, Garcia Jr G, Arumugaswami V, Wirz RE. Cold atmospheric plasma for SARS-CoV-2 inactivation. Physics of Fluids. 2020;32(11):111702.
- Goldman SA. Stem and progenitor cell-based therapy of the central nervous system: hopes, hype, and wishful thinking. Cell stem cell. 2016;18(2):174-88.
- Weltmann K, Von Woedtke T. Plasma medicine—current state of research and medical application. Plasma Physics and Controlled Fusion. 2016;59(1):014031.
- Jang J-Y, Hong YJ, Lim J, Choi JS, Choi EH, Kang S, et al. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials. 2018;156:258-73.
- Xiong Z, Zhao S, Mao X, Lu X, He G, Yang G, et al. Selective neuronal differentiation of neural stem cells induced by nanosecond microplasma agitation. Stem Cell Research. 2014;12(2):387-99.
- Nam M-K, Kim G-Y, Yun S-E, Jang J-Y, Kim Y-H, Choi EH, et al. Harmless effects of argon plasma on caudal fin regeneration and embryogenesis of zebrafish: novel biological approaches for safe medical applications of bioplasma. Experimental & molecular medicine. 2017;49(7):e355-e.
- Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9(11):735.
- Park J, Lee H, Lee HJ, Kim GC, Kim S-S, Han S, et al. Non-thermal atmospheric pressure plasma is an excellent tool to activate proliferation in various mesoderm-derived human adult stem cells. Free Radical Biology and Medicine. 2019;134:374-84.
- Aikman B, De Almeida A, Meier-Menches SM, Casini A. Aquaporins in cancer development: Opportunities for bioinorganic chemistry to contribute novel chemical probes and therapeutic agents. Metallomics. 2018;10(5):696-712.
- Yusupov M, Razzokov J, Cordeiro RM, Bogaerts A. Transport of reactive oxygen and nitrogen species across aquaporin: A molecular level picture. Oxidative medicine and cellular longevity. 2019;2019.
- Tamma G, Valenti G, Grossini E, Donnini S, Marino A, Marinelli RA, et al. Aquaporin membrane channels in oxidative stress, cell signaling, and aging: recent advances and research trends. Oxidative medicine and cellular longevity. 2018;2018.
- Rivel T, Ramseyer C, Yesylevskyy S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Scientific reports. 2019;9(1):1-14.
- Van der Paal J, Neyts EC, Verlackt CC, Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chemical science. 2016;7(1):489-98.
- Keidar M, Yan D, Beilis II, Trink B, Sherman JH. Plasmas for treating cancer: Opportunities for adaptive and self-adaptive approaches. Trends in biotechnology. 2018;36(6):586-93.
- Wiegand C, Fink S, Beier O, Horn K, Pfuch A, Schimanski A, et al. Dose-and time-dependent cellular effects of cold atmospheric pressure plasma evaluated in 3D skin models. Skin pharmacology and physiology. 2016;29(5):257-65.
- Yan D, Talbot A, Nourmohammadi N, Cheng X, Canady J, Sherman J, et al. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Scientific reports. 2015;5(1):1-17.
- Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta S, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. British journal of cancer. 2011;105(9):1295-301.
- Kaushik N, Uddin N, Sim GB, Hong YJ, Baik KY, Kim CH, et al. Responses of solid tumor cells in DMEM to reactive oxygen species generated by non-thermal plasma and chemically induced ROS systems. Scientific reports. 2015;5(1):1-11.
- Cha J-Y, Lee H-J. Targeting lipid metabolic reprogramming as anticancer therapeutics. Journal of cancer prevention. 2016;21(4):209.
- Xu D, Ning N, Xu Y, Wang B, Cui Q, Liu Z, et al. Effect of cold atmospheric plasma treatment on the metabolites of human leukemia cells. Cancer Cell International. 2019;19(1):1-12.
- Gjika E, Pal-Ghosh S, Kirschner ME, Lin L, Sherman JH, Stepp MA, et al. Combination therapy of cold atmospheric plasma (CAP) with temozolomide in the treatment of U87MG glioblastoma cells. Scientific Reports. 2020;10(1):1-13.
- Alimohammadi M, Golpour M, Sohbatzadeh F, Hadavi S, Bekeschus S, Niaki HA, et al. Cold atmospheric plasma is a potent tool to improve chemotherapy in melanoma in vitro and in vivo. Biomolecules. 2020;10(7):1011.
- Lin L, Ding C-B, Jin T, Han X-H, Zhou H, Wu Z-W, et al. A meaningful attempt: Applying dielectric barrier discharge plasma to induce apoptosis of MDA-MB-231 cells via regulating HIF-1α/VEGFA expression. Surface and Coatings Technology. 2020;401:126293.
- Chen Z, Simonyan H, Cheng X, Gjika E, Lin L, Canady J, et al. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers. 2017;9(6):61.
- Chen Z, Lin L, Zheng Q, Sherman JH, Canady J, Trink B, et al. Micro-sized cold atmospheric plasma source for brain and breast cancer treatment. Plasma Medicine. 2018;8(2).
- Mirpour S, Piroozmand S, Soleimani N, Jalali Faharani N, Ghomi H, Fotovat Eskandari H, et al. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Scientific reports. 2016;6(1):1-10.
- Mashayekh S, Rajaee H, Akhlaghi M, Shokri B, Hassan ZM. Atmospheric-pressure plasma jet characterization and applications on melanoma cancer treatment (B/16-F10). Physics of Plasmas. 2015;22(9):093508.
- Semmler ML, Bekeschus S, Schäfer M, Bernhardt T, Fischer T, Witzke K, et al. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (CAP) in cancer treatment. Cancers. 2020;12(2):269.
- Manaloto E, Gowen AA, Lesniak A, He Z, Casey A, Cullen PJ, et al. Cold atmospheric plasma induces silver nanoparticle uptake, oxidative dissolution and enhanced cytotoxicity in glioblastoma multiforme cells. Archives of Biochemistry and Biophysics. 2020;689:108462.
- Mihai C-T, Mihaila I, Pasare MA, Pintilie RM, Ciorpac M, Topala I. Cold Atmospheric Plasma-Activated Media Improve Paclitaxel Efficacy on Breast Cancer Cells in a Combined Treatment Model. Current Issues in Molecular Biology. 2022;44(5):1995-2014.
- Ninomiya K, Ishijima T, Imamura M, Yamahara T, Enomoto H, Takahashi K, et al. Evaluation of extra-and intracellular OH radical generation, cancer cell injury, and apoptosis induced by a non-thermal atmospheric-pressure plasma jet. Journal of Physics D: Applied Physics. 2013;46(42):425401.
- Kim C-H, Bahn JH, Lee S-H, Kim G-Y, Jun S-I, Lee K, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. Journal of biotechnology. 2010;150(4):530-8.
- Ishaq M, Evans MD, Ostrikov KK. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2–ASK1 apoptosis pathways and oxidative stress is mitigated by Srx–Nrf2 anti-oxidant system. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2014;1843(12):2827-37.
- Plewa J-M, Yousfi M, Frongia C, Eichwald O, Ducommun B, Merbahi N, et al. Low-temperature plasma-induced antiproliferative effects on multi-cellular tumor spheroids. New Journal of Physics. 2014;16(4):043027.
- Ahn HJ, Kim KI, Hoan NN, Kim CH, Moon E, Choi KS, et al. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PloS one. 2014;9(1):e86173.
- Tanaka H, Mizuno M, Ishikawa K, Toyokuni S, Kajiyama H, Kikkawa F, et al. Cancer treatments using low-temperature plasma. Current Medicinal Chemistry. 2021;28(41):8549-58.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017;541(7637):321-30.
- Tanaka H, Mizuno M, Ishikawa K, Toyokuni S, Kajiyama H, Kikkawa F, et al. New hopes for plasma-based cancer treatment. Plasma. 2018;1(1).
- Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: Implications for cancer immunotherapy. Biochemical pharmacology. 2018;153:12-23.
- Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35(46):5931-41.
- Guerrero-Preston R, Ogawa T, Uemura M, Shumulinsky G, Valle BL, Pirini F, et al. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. International journal of molecular medicine. 2014;34(4):941-6.
- Hasse S, Seebauer C, Wende K, Schmidt A, Metelmann H-R, von Woedtke T, et al. Cold argon plasma as adjuvant tumour therapy on progressive head and neck cancer: A preclinical study. Applied Sciences. 2019;9(10):2061.
- Kim K, Jun Ahn H, Lee J-H, Kim J-H, Sik Yang S, Lee J-S. Cellular membrane collapse by atmospheric-pressure plasma jet. Applied Physics Letters. 2014;104(1):013701.
- Torii K, Yamada S, Nakamura K, Tanaka H, Kajiyama H, Tanahashi K, et al. Effectiveness of plasma treatment on gastric cancer cells. Gastric cancer. 2015;18(3):635-43.
- Privat-Maldonado A, Verloy R, Cardenas Delahoz E, Lin A, Vanlanduit S, Smits E, et al. Cold atmospheric plasma does not affect stellate cells phenotype in pancreatic cancer tissue in Ovo. International journal of molecular sciences. 2022;23(4):1954.
- Choi J-H, Gu H-J, Park K-H, Hwang D-S, Kim G-C. Anti-Cancer Activity of the Combinational Treatment of Noozone Cold Plasma with p-FAK Antibody-Conjugated Gold Nanoparticles in OSCC Xenograft Mice. Biomedicines. 2022;10(9):2259.
- Lu Y, Song H, Bai F, Li L, Wang Q, Chen G, et al. Cold atmospheric plasma for cancer treatment: molecular and immunological mechanisms. IEEE Transactions on Radiation and Plasma Medical Sciences. 2022.
- Erfani R, Carmichael C, Sofokleous T, Wang Q. Nanosecond-pulsed DBD plasma treatment on human leukaemia Jurkat cells and monoblastic U937 cells in vitro. Scientific reports. 2022;12(1):1-13.
- Adachi T, Matsuda Y, Ishii R, Kamiya T, Hara H. Ability of plasma-activated acetated Ringer’s solution to induce A549 cell injury is enhanced by a pre-treatment with histone deacetylase inhibitors. Journal of Clinical Biochemistry and Nutrition. 2020;67(3):232-9.
- Laroussi M. Plasma medicine: a brief introduction. Plasma. 2018;1(1):47-60.
- Holcomb JD. Plasma energy skin rejuvenation. Facial Plastic Surgery Clinics. 2020;28(1):67-74.
- Isbary G, Morfill G, Schmidt H, Georgi M, Ramrath K, Heinlin J, et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. British Journal of Dermatology. 2010;163(1):78-82.
- Guo J, Huang Y, Xu B, Yang J. Efficacy of Cold Atmospheric Plasma Therapy on Chronic Wounds: An Updated Systematic Review and Meta-Analysis of RCTs. Computational and Mathematical Methods in Medicine. 2022;2022.
- Lee Y, Lee MH, Phillips SA, Stacey MC. Growth factors for treating chronic venous leg ulcers: A systematic review and meta‐analysis. Wound Repair and Regeneration. 2022;30(1):117-25.
- Elsharnoby AM, El-Barbary AH, Eldeeb AE, Hassan HA. Resistant Chronic Venous Leg Ulcers: Effect of Adjuvant Systemic Hyperbaric Oxygen Therapy Versus Venous Intervention Alone. The International Journal of Lower Extremity Wounds. 2022:15347346221100891.
- Kavitha H, Kumari A. A Study of diabetic foot ulcer with multidrug-resistant organism infection.
- Brehmer F, Haenssle H, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, et al. Alleviation of chronic venous leg ulcers with a hand‐held dielectric barrier discharge plasma generator (PlasmaDerm® VU‐2010): results of a monocentric, two‐armed, open, prospective, randomized and controlled trial (NCT 01415622). Journal of the European Academy of Dermatology and Venereology. 2015;29(1):148-55.
- Chuangsuwanich A, Assadamongkol T, Boonyawan D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: a randomized controlled trial. The international journal of lower extremity wounds. 2016;15(4):313-9.
- Chatraie M, Torkaman G, Khani M, Salehi H, Shokri B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Scientific reports. 2018;8(1):1-11.
- Gao J, Wang L, Xia C, Yang X, Cao Z, Zheng L, et al. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. International Wound Journal. 2019;16(5):1103-11.
- Ulrich C, Kluschke F, Patzelt A, Vandersee S, Czaika V, Richter H, et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. Journal of wound care. 2015;24(5):196-203.
- Emmert S, Bernhardt T, Schäfer M, Semmler ML, Bekeschus S, Masur K, et al. Cold Plasma Treatment for Chronic Wounds. Textbook of Good Clinical Practice in Cold Plasma Therapy: Springer; 2022. p. 141-60.
- von Woedtke T, Schmidt A, Bekeschus S, Wende K, Weltmann K-D. Plasma medicine: A field of applied redox biology. In Vivo. 2019;33(4):1011-26.
- Xiong Z. Cold atmospheric pressure plasmas (CAPs) for skin wound healing. Plasma Medicine-Concepts and Clinical Applications. 2018:121-33.
- Schmidt A, Bekeschus S, Wende K, Vollmar B, von Woedtke T. A cold plasma jet accelerates wound healing in a murine model of full‐thickness skin wounds. Experimental dermatology. 2017;26(2):156-62.
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric‐pressure plasma technology in the treatment of keloid: A randomized controlled trial. Journal of Cosmetic Dermatology. 2022.
- Schmidt A, von Woedtke T, Bekeschus S. Periodic exposure of keratinocytes to cold physical plasma: an in vitro model for redox-related diseases of the skin. Oxidative medicine and cellular longevity. 2016;2016.
- Schmidt A, Bekeschus S, Jarick K, Hasse S, von Woedtke T, Wende K. Cold physical plasma modulates p53 and mitogen-activated protein kinase signaling in keratinocytes. Oxidative Medicine and Cellular Longevity. 2019;2019.
- Shome D, von Woedtke T, Riedel K, Masur K. The HIPPO transducer YAP and its targets CTGF and Cyr61 drive a paracrine signalling in cold atmospheric plasma-mediated wound healing. Oxidative medicine and cellular longevity. 2020;2020.
- Sun Y, Hu L, Tao Z, Jarugumilli GK, Erb H, Singh A, et al. Pharmacological blockade of TEAD–YAP reveals its therapeutic limitation in cancer cells. Nature Communications. 2022;13(1):1-18.
- Duchesne C, Banzet S, Lataillade JJ, Rousseau A, Frescaline N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. The Journal of pathology. 2019;249(3):368-80.
- Lee OJ, Ju HW, Khang G, Sun PP, Rivera J, Cho JH, et al. An experimental burn wound‐healing study of non‐thermal atmospheric pressure microplasma jet arrays. Journal of Tissue Engineering and Regenerative Medicine. 2016;10(4):348-57.
- Burducea I, Burducea C, Mereuta P-E, Sirbu S-R, Iancu D-A, Istrati M-B, et al. Helium Atmospheric Pressure Plasma Jet Effects on Two Cultivars of Triticum aestivum L. Foods. 2023;12(1):208.
- Betancourt-Ángeles M, Peña-Eguiluz R, López-Callejas R, Domínguez-Cadena NA, Mercado-Cabrera A, Muñoz-Infante J, et al. Treatment in the healing of burns with a cold plasma source. International Journal of Burns and Trauma. 2017;7(7):142.
- van Welzen A, Hoch M, Wahl P, Weber F, Rode S, Tietze JK, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: A controlled pilot study. Skin pharmacology and physiology. 2021;34(6):328-36.
- Winter S, Meyer-Lindenberg A, Wolf G, Reese S, Nolff MC. In vitro evaluation of the decontamination effect of cold atmospheric argon plasma on selected bacteria frequently encountered in small animal bite injuries. Journal of microbiological methods. 2020;169:105728.
- Winter S, Nolff MC, Reese S, Meyer-Lindenberg A. Vergleich Der Effizienz von Polyhexanid-Biguanid, Argon-Kaltplasma Und Kochsalzlavage Zur Dekontamination von Bisswunden Beim Hund. Tierärztliche Praxis Ausgabe K: Kleintiere/Heimtiere. 2018;46(02):73-82.
- Nishijima A, Fujimoto T, Hirata T, Nishijima J. Effects of cold atmospheric pressure plasma on accelerating acute wound healing: a comparative study among 4 different treatment groups. Modern Plastic Surgery. 2019;9(01):18.
- Rutkowski R, Daeschlein G, von Woedtke T, Smeets R, Gosau M, Metelmann H-R. Long-term risk assessment for medical application of cold atmospheric pressure plasma. Diagnostics. 2020;10(4):210.
- Park J, Lee H, Lee HJ, Kim GC, Kim DY, Han S, et al. Non-thermal atmospheric pressure plasma efficiently promotes the proliferation of adipose tissue-derived stem cells by activating NO-response pathways. Scientific reports. 2016;6(1):1-12.
- Elsaadany M, Subramanian G, Ayan H, Yildirim-Ayan E. Exogenous nitric oxide (NO) generated by NO-plasma treatment modulates osteoprogenitor cells early differentiation. Journal of Physics D: Applied Physics. 2015;48(34):345401.
- Han I, Choi EH. The role of non-thermal atmospheric pressure biocompatible plasma in the differentiation of osteoblastic precursor cells, MC3T3-E1. Oncotarget. 2017;8(22):36399.
- Klotz L-O, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox biology. 2015;6:51-72.
- Zare S, Shirkavand A, Eslami E, Fathollah S, Mansouri P. Biophysical evaluation of treating adipose tissue-derived stem cells using non-thermal atmospheric pressure plasma. Scientific Reports. 2022;12(1):1-13.
- Xiong Z, Zhao S, Yan X. Nerve stem cell differentiation by a one-step cold atmospheric plasma treatment in vitro. JoVE (Journal of Visualized Experiments). 2019(143):e58663.
- Alfano A, Xu J, Yang X, Deshmukh D, Qiu Y. SRC Kinase-Mediated Tyrosine Phosphorylation of TUBB3 Regulates Its Stability and Mitotic Spindle Dynamics in Prostate Cancer Cells. Pharmaceutics. 2022;14(5):932.
- Lazzarini R, Guarnieri S, Fulgenzi G, Mariggiò MA, Graciotti L, Martiniani M, et al. Mesenchymal stem cells from nucleus pulposus and neural differentiation potential: a continuous challenge. Journal of Molecular Neuroscience. 2019;67(1):111-24.
- Bourdens M, Jeanson Y, Taurand M, Juin N, Carrière A, Clément F, et al. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Scientific reports. 2019;9(1):1-15.
- Ranjan R, Krishnamraju P, Shankar T, Gowd S. Nonthermal plasma in dentistry: an update. Journal of International Society of Preventive & Community Dentistry. 2017;7(3):71.
- Wen Y, Luo Y, Wei X, Tan H, Ai R, Xiong Z, et al. Antibacterial effects of liquid discharge cold plasma on Enterococcus faecalis planktonic cultures and biofilms: an in vitro study of root canal treatment. Journal of Physics D: Applied Physics. 2022;55(36):365204.
- Marsh P, Zaura E. Dental biofilm: ecological interactions in health and disease. Journal of clinical periodontology. 2017;44:S12-S22.
- Delben JA, Zago CE, Tyhovych N, Duarte S, Vergani CE. Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PloS one. 2016;11(5):e0155427.
- Armand A, Khani M, Asnaashari M, AliAhmadi A, Shokri B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagnosis and Photodynamic Therapy. 2019;26:327-33.
- Koosha F, Cymerman J, Manders T, Simon M, Walker S, Rafailovich M. Introducing a non-cytotoxic root canal dressing with improved antimicrobial efficacy. Journal of Endodontics. 2022.
- Shahmohammadi Beni M, Han W, Yu K. Dispersion of OH radicals in applications related to fear-free dentistry using cold plasma. Applied Sciences. 2019;9(10):2119.
- Dong X, Chen M, Wang Y, Yu Q. A mechanistic study of plasma treatment effects on demineralized dentin surfaces for improved adhesive/dentin interface bonding. Clinical plasma medicine. 2014;2(1):11-6.
- Xiong B, Chai Y, Yuan S, Niu G. Effects of plasma-induced grafting modification on the adhesive strength and mechanical properties of fiber posts. Eur Rev Med Pharmacol Sci. 2022;26(21):7840-9.
- Yang Y, Guo J, Zhou X, Liu Z, Wang C, Wang K, et al. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: An in vitro study. Dental Materials Journal. 2018;37(1):157-66.
- Monetto I. The effects of an interlayer debond on the flexural behavior of three-layer beams. Coatings. 2019;9(4):258.
- Yang Y, Zheng M, Li J, Su Y-F, Li H-P, Tan J-G. Inhibition of bacterial growth on zirconia abutment with a helium cold atmospheric plasma jet treatment. Clinical Oral Investigations. 2020;24(4):1465-77.
- Jiang C, Miles J, Hornef J, Carter C, Adams S. Electron densities and temperatures of an atmospheric-pressure nanosecond pulsed helium plasma jet in air. Plasma Sources Science and Technology. 2019;28(8):085009.
- Tanaka H, Ishikawa K, Mizuno M, Toyokuni S, Kajiyama H, Kikkawa F, et al. State of the art in medical applications using non-thermal atmospheric pressure plasma. Reviews of Modern Plasma Physics. 2017;1(1):1-89.
- Vasilets VN, Shekhter AB, Guller AE, Pekshev AV. Air plasma-generated nitric oxide in treatment of skin scars and articular musculoskeletal disorders: Preliminary review of observations. Clinical Plasma Medicine. 2015;3(1):32-9.
- Tiwari S, Avinash A, Katiyar S, Iyer AA, Jain S. Dental applications of ozone therapy: A review of literature. The Saudi Journal for Dental Research. 2017;8(1-2):105-11.
- Weiss M, Utz R, Ackermann M, Taran FA, Krämer B, Hahn M, et al. Characterization of a non‐thermally operated electrosurgical argon plasma source by electron spin resonance spectroscopy. Plasma Processes and Polymers. 2019;16(2):1800150.
- Adamovich I, Baalrud S, Bogaerts A, Bruggeman P, Cappelli M, Colombo V, et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. Journal of Physics D: Applied Physics. 2017;50(32):323001.
- Mann MS, Tiede R, Gavenis K, Daeschlein G, Bussiahn R, Weltmann K-D, et al. Introduction to DIN-specification 91315 based on the characterization of the plasma jet kINPen® MED. Clinical Plasma Medicine. 2016;4(2):35-45.
- Judée F, Dufour T. Plasma gun for medical applications: engineering an equivalent electrical target of the human body and deciphering relevant electrical parameters. Journal of Physics D: Applied Physics. 2019;52(16):16LT02.
- Lackmann J-W, Wende K, Verlackt C, Golda J, Volzke J, Kogelheide F, et al. Chemical fingerprints of cold physical plasmas–an experimental and computational study using cysteine as tracer compound. Scientific reports. 2018;8(1):1-14.
- Hahn V, Brandenburg R, von Woedtke T. DIN SPEC 91315: A first attempt to implement mandatory test protocols for the characterization of plasma medical devices. Comprehensive Clinical Plasma Medicine: Springer; 2018. p. 511-6.
- Setsuhara Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Archives of biochemistry and biophysics. 2016;605:3-10.
- Winter J, Brandenburg R, Weltmann K. Atmospheric pressure plasma jets: an overview of devices and new directions. Plasma Sources Science and Technology. 2015;24(6):064001.
- Hirst AM, Frame FM, Arya M, Maitland NJ, O’Connell D. Low temperature plasmas as emerging cancer therapeutics: the state of play and thoughts for the future. Tumor Biology. 2016;37(6):7021-31.
- Jo A, Joh HM, Bae JH, Kim SJ, Chung TH, Chung JW. Plasma activated medium prepared by a bipolar microsecond-pulsed atmospheric pressure plasma jet array induces mitochondria-mediated apoptosis in human cervical cancer cells. Plos one. 2022;17(8):e0272805.
- Chen Z, Chen G, Obenchain R, Zhang R, Bai F, Fang T, et al. Cold atmospheric plasma delivery for biomedical applications. Materials Today. 2022.
- Nastuta AV, Gerling T. Cold atmospheric pressure plasma jet operated in Ar and He: From basic plasma properties to vacuum ultraviolet, electric field and safety thresholds measurements in plasma medicine. Applied Sciences. 2022;12(2):644.
- Hamouda SA, Alshawish NK, Abdalla YK, Ibrahim MK. Ultraviolet Radiation: Health Risks and Benefits. Saudi J Eng Technol. 2022;7(10):533-41.
- Sailer U. kINPen® MED–The Precise Cold Plasma Jet. Textbook of Good Clinical Practice in Cold Plasma Therapy: Springer; 2022. p. 281-305.
- Lee H, Park S, Park JY, Kim J, Choe W. Ultraviolet Light-Driven Gaining of Hydroxyl and Nitrogen Oxide Radicals in Plasma–Treated Water. Chemical Engineering Journal. 2023:141425.
- Jia P, Chen M, Jia H, Tan X, Ran J, Wu K, et al. Ozone concentration measurement of an atmospheric pressure air plasma jet by ultra-violet absorption spectroscopy. IEEE Transactions on Radiation and Plasma Medical Sciences. 2022.
- Nam S, Um W. Decontamination of radioactive metal wastes using underwater microwave plasma. Journal of Environmental Chemical Engineering. 2022;10(1):107090.
- Isbary G, Köritzer J, Mitra A, Li Y-F, Shimizu T, Schroeder J, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clinical Plasma Medicine. 2013;1(1):36-44.

 ETFLIN
Notification
ETFLIN
Notification











