A Review Focusing on the Benefits of Green Tea Catechins as Nutraceuticals
by Namrata Santosh Naware ★ , Shreya Sakharam Ambatkar , Tanmay Sanjay Kamble , Sonal Bangar , Kiran Babu Uppar , Kshitij Shirke , Mukesh Patil , Ashish Jain
Academic editor: James H. Zothantluanga
Sciences of Phytochemistry 2(2): 138-146 (2023); https://doi.org/10.58920/sciphy02020001
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
29 Apr 2023
12 Jun 2023
01 Jul 2023
01 Jul 2023
Abstract: Any product generated from food sources that offer additional health advantages over and above the essential nutritional content present in foods is referred to as a nutraceutical under the broad umbrella term. The catechins in Camellia sinensis (Theaceae) namely (-)-epicatechin, (-)-epicatechin-3-gallate, (-)-epigallocatechin, and (-)-epigallocatechin-3-gallate (EGCG), which can be used as nutraceuticals in food or as a component of food items has been discussed. Catechins being polyphenols and antioxidants are found to have a wide range of therapeutic application like weight loss, anticancer, anti-inflammatory, and a few more therapeutic applications, through various mechanisms like stimulating AMP-activated protein kinase, enhanced apoptosis, decreased expression of interleukin (IL)-6 and IL-8. The most recent method for extracting catechins include combining the extraction processing of PEF or IPL with Subcritical water extraction. Food additives have been mixed with green tea extracts to develop a desired formulation like chewing gum and capsule. As green tea catechins are a beneficial phytoconstituents to improve overall health, its prospects include formulation of a gelatin gummy formulation which will improve its palatability by masking the bitter taste. Gelatin gummy formulation can be carried conveniently and will provide easy access to the consumer as compared to green tea. Furthermore, we found a scope to develop an analytical method for EGCG and carry out its validation by HPLC which will be more reliable and cost-efficient in comparison to the existing UHPLC methods for EGCG.
Keywords: Camellia sinensisEpicatechinEpicatechin-3-gallateEpigallocatechin-3-gallateNutraceutical
Introduction
Nutraceuticals are defined as "specially tailored formulations" created to meet certain dietary needs and/or provide preventive healthcare. Dr. Stephen De Felice coined the phrase "nutraceutical" in 1989, combining the words "nutrition" and "pharmaceutical." These are foods or components of foods that have a variety of health advantages, such as the ability to treat and/ or prevent disease (1).
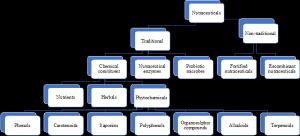
The classification of Nutraceuticals is given in Figure 1. The transition to nutraceuticals is being driven by a number of factors such as:
- More and more people are worried about the cost of healthcare (2).
- Those dissatisfied with pharmaceutical agents in promoting health are switching to nutraceuticals to improve their health and to prevent chronic disease (2).
- The fact that our overly processed food supply, which is made from crops farmed with chemical fertilizers, pesticides, herbicides, and frequently genetically engineered seeds, lacks the required nutrients for maximum health is acknowledged by healthcare providers (2).
- People place a higher priority on prevention rather than treatment (2).
- People with chronic conditions for whom allopathic therapy has proven ineffective (2).
- Financially disadvantaged patients (2).

Figure 1. Classification of nutraceuticals.

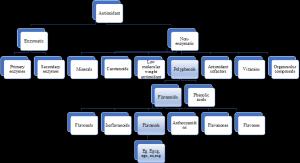
Figure 2. Classification of antioxidant.
There is some overlap between the phrases functional food, dietary supplements, and nutraceuticals. Over time, specific definitions and requirements for these items have been supplied by the laws and regulations in this field, always keeping safety considerations in mind (3). The Dietary Supplement and Health Education Act (DSHEA), which was revised in the United States in 1994, provides a road map for the registration of nutraceuticals and dietary supplements in the nation for marketing purposes. In India, the Food Safety Standards Act of 2006 and the Food Safety Standard Rules and Regulations of 2011 are in place to prevent the classification of dietary supplements as either food or drugs (4).
The last ten years have seen a surge in interest in the antioxidant properties of dietary plant polyphenols as we can see the classification of antioxidants in Figure 2. Long-term intake of diets high in plant polyphenols may protect against the onset of malignancies, cardiovascular disease, diabetes, osteoporosis, and neurological illnesses, according to epidemiological research and related meta-analyses (5).
The dietary sources of polyphenols have drawn a lot of interest. Particularly, the polyphenolic elements in various tea brews have been thoroughly investigated. After water, tea is the beverage that is consumed most frequently. Due to the strong correlation between drinking tea and having positive health effects, tea polyphenols have drawn a lot of public interest. Epigallocatechin-gallate (EGCG), epigallocatechin (EGC), epicatechin-gallate (ECG), and epicatechin (EC) are among the catechins found in green tea extract. The most abundant polyphenol in green tea is EGCG (6). One of the most numerous and extensively dispersed classes of natural compounds in the plant kingdom is dietary phenolics, often known as polyphenols. Almost 4000 flavonoids have been discovered among more than 8000 phenolic structures currently known. While being classified chemically as substances having phenolic structural characteristics, polyphenols are a wide class of natural products that include many subgroups of phenolic substances. Rich sources of polyphenols include fruits, vegetables, whole grains, and various types of meals and beverages like tea, chocolate, and wine (7).
Pharmacognosy of Green Tea
Synonym of EGCG
Epigallocatechin-3-gallate; (–)-epigallocatechin-3-O-gallate (8).
Biological Source
Epigallocatechin gallate is the major polyphenolic component of dried C. sinensis belonging to the family Theaceae. EGCG is the biologically active catechin accounting for at least 50% of the total catechin content in C. sinensis leaves (9).
Geographical Indications
The tea tree, C. sinensis, is thought to have started in an evergreen forest. According to the report, large-scale tea tree cultivation is concentrated mostly in the plateau sections of Japan's Kyushu and Honshu Islands. China accounts for over 73% of the global output of green tea. The production of green tea in Vietnam and Indonesia, which together account for about 5% of global production, is especially noteworthy. India is one of the world's top producers of green tea (10).

Figure 3. A picture of C. sinensis leaves.
Microscopy
The leaf has calcium oxalate crystal-containing heterogenous mesophyll, actinocytic stomata, unicellular trichomes, and sclereid cells (11).
Extraction of Green Tea
The development of an appropriate method for the extraction, separation, and stability of catechin concerns scientists and industry very much. Several key factors have a considerable impact on catechin extraction, including pH, temperature, frequency, and timing of extractions, and the type of solvent employed. This is a discussion of some popular methods for extracting catechin from green tea leaves (12).
The efficiency of pulsed electric field (PEF) and Intense Pulsed Light (IPL) as a pre-treatment method for improving the extraction of tea catechins from green tea leaves was confirmed by Hee-Jeong Hwang et al. (2021) by combining the extraction processing of PEF or IPL with Subcritical water extraction (SWE) (13).
SWE is a technique for retaining water in a liquid condition for extraction at a temperature between 100 °C (the boiling point of water) and 374 °C (the critical point of water) while elevating the boiling point of water in a high-pressure environment (about 10 MPa) (14). Similar to organic solvents, the polarity of water shifts from polar to nonpolar as the temperature rises, making it easier to dissolve non-polar and moderately polar compounds (13).
The PEF technique involves submerging a sample with two electrodes in water or solvents and delivering high-voltage electric pulses for a brief period time. PEF has historically been employed primarily as a non-thermal sterilization technique, however, it is currently used as an extraction technique in and of itself or to increase extraction effectiveness (15).
One of the newer non-thermal disinfection techniques that use intense, brief bursts of light is called IPL. However, current research from multiple studies has shown that after IPL treatment of the plant surface, the extraction efficiency of health-promoting chemicals from plants is boosted (16).
Phytochemistry of Green Tea Catechins
Tea leaves' chemistry has a well-established history. Caffeine (about 3.5% of the dry weight of fresh tea leaves), theobromine (0.15-0.2%), theophylline (0.02-0.04%), and other methylxanthines, along with lignin (6.5%), organic acids (1.5%), chlorophyll (0.5%), and other pigments, theanine (4%), free amino acids (1-5.5%), and various flavouring compounds are all present in fresh tea leaves. There are also a large number of other substances, such as flavones, phenolic acids, depsides, carbohydrates, alkaloids, minerals, vitamins, and enzymes. Flavonols, primarily quercetin, kaempferol, and myricetin, and their glycosides are also present in tea (17).
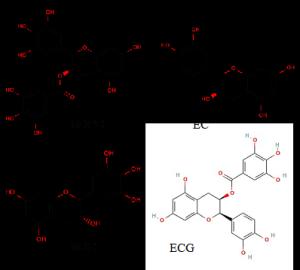
Even while numerous catechins are found in trace amounts, EGCG is by far the most prevalent green tea polyphenol by weight. It consists of a pyrogallol ring (ring A), a benzenediol ring (ring B), a galloyl group, and a tetrahydropyran moiety (ring C) (with the B ring) (18, 19) as shown in Figure 4. The other catechins present are ECG, which lacks a hydroxyl group on the pyrogallol B ring, and EGC (20).
The structural features of green tea catechins that significantly contribute to their biological action are the number and positions of the hydroxyl groups (or their substituents) on the rings, which determine their ability to interact with biological matter through hydrogen bonding, or electron and hydrogen transfer processes within their anti-oxidant activities, and by the presence/absence of the galloyl moiety, which clearly distinguishes EGC from the other three catechins (21).
The B- and D-rings are connected to a decrease in proteasome activity. These protected analogs also caused apoptotic cell death in a way that was particular to tumor cells. According to the literature anti-cancer and cancer-prevention drugs could be created using the B-ring/D-ring peracetate-protected EGCG analogues. According to the data, phenolic groups on the A-ring helps to inhibit the heat-shock protein 90 (Hsp90), whereas phenolic substituents on the D-ring are harmful. Finally, the hydroxyl group at the 5'-position in the B-ring inhibits the stomach's Helicobacter pylori bacteria and demonstrated 35-104-fold urease inhibition when compared to catechins without the group (8).
The formal condensation of gallic acid with the (3R)-hydroxy group of epigallocatechin results in epigallocatechin 3-gallate, a gallate ester with the chemical formula C22H18O11 and molecular weight 458.4. It functions as an antioxidant, an Hsp90 inhibitor, an antineoplastic agent, a neuroprotective agent, a plant metabolite, a geroprotector, and an inducer of apoptosis in carcinoma cells.
The addition of EGCG can prevent the generation of ROS and other photosensitized redox reactions that lead to the oxidation of EC in response to blue light illumination. Yet, due to its chemical reactivity, it can easily produce reactive oxygen species (ROS) by auto-oxidation and display pro-oxidant effects, which can be both good and bad, when used in large doses (18, 22).

Figure 4. Structure of catechins present in green tea (23, 24, 25, 26).
Pharmacokinetics of Green Tea Catechins
EGCG is quickly absorbed by the intestinal system, disseminated, metabolized in the liver and colon, and can be reabsorbed from the gut by enterohepatic re-circulation, according to studies on ADME in rats and dogs. The resultant metabolites are eliminated by the urine and biliary systems. However, after oral delivery, only minute amounts of EGCG are found in the urine (8). Green tea catechins mostly enter the body through the jejunum and ileum, where they are diffused through epithelial cells in a para-cellular manner (27). After absorption, EGCG is present in plasma in a significant amount (>75%) in a free form (28).
Pharmacology of Green Tea Catechins
Green tea catechins (GTC) work by lowering oxidative stress, preventing inflammatory events, reducing platelet aggregation, and stopping the growth of vascular smooth muscle cells to prevent atherosclerosis, hypertension, endothelial dysfunction, ischemic heart diseases, cardiomyopathy, cardiac hypertrophy, and congestive heart failure by raising NO levels (29), GTC also had anti-atherosclerotic effects on smokers' defective vasculature (30). Several in vitro and in vivo experimental investigations have demonstrated the effectiveness of tea's bioactive components in preventing cardiovascular diseases. More than one cup of green tea every day lowers the risk of coronary heart disease by 10% (31). EGC decreased the synthesis of IL-8 in human ECs, which can lessen the atherosclerosis brought on by inflammation (32).
According to the study conducted by Antonello M., et al. (2007). Male Sprague Dawley rats aged 13 were given a vehicle, a high (700 g/kg/d) or a low (350 g/kg/d) Angiotensin II dose for 13 days and were randomly allocated to drink water with or without green tea extract which likely inhibited superoxide anion production or scavenged it to prevent hypertension and target organ damage brought on by a high Angiotensin II dosage (33).
In mice, GTC and EGCG delayed the tail bleeding time, reduced death from pulmonary thrombosis, and inhibited human platelet aggregation in vitro and ex vivo (34).
A total of 12 weeks of treatment with high doses of green tea extract helped women with central obesity lose considerable amounts of weight, shrink their waistlines, and consistently lower their levels of LDL and total cholesterol in their blood (35).
According to the study conducted by Li G, Yang J., et al. (2020), one group functioned as the control group, and the other two groups namely CUMS (Chronic Unpredictable Mild Stress) group and CUMS+EGCG group, respectively were subjected to chronic unpredictable mild stress intervention. A total of 24 stochastically selected rats were separated into three groups. The investigation results of this experimental study showed that the intervention of EGCG treatment might have significant implications in several areas, including anti-depression, regulation of 5-HT concentration, enhancement of intestinal hyper-permeability, and neuroprotection in the hippocampus. (36)
Before and after receiving 300 mg of EGCG or a matching placebo, participants underwent baseline tests of their cardiovascular, cognitive, and emotional health as well as an electroencephalogram (EEG) in the resting state. After taking EGCG, participants in the EGCG condition may have felt more at ease and attentive (37)
The dextran sulfate sodium (DSS)-treated mice model of ulcerative colitis was used to study the effects of EGCG. For three days, only EGCG (3.2 mg/g) was consumed. Treatment with EGCG reduced spleen and colon lengthening brought on by DSS. When compared to DSS-treated controls, EGCG also reduced the levels of the proteins IL-1, IL-6, and tumor necrosis factor, as well as colonic lipid peroxides (38).
Table 1. Green tea catechin formulations application dose and content.
No. | Formulation | Content and Dose | Application | Reference |
1 | Chewing gum | Caffeine, catechin, and flavonoid of the hydroalcoholic extraction were 207.32 mg/g, 130.00 mg/g, and 200.82 mg/g, respectively | Antioxidant | (40) |
2 | Capsule | Green tea polyphenols 250mg | Antioxidant | (41) |
3 | Soy-fortified green tea curd
| - | Lactose intolerance, hypertension, hypercholesterolemia, malnutrition | (42) |
4 | Protein-reinforced alginate hydrogel beads for the encapsulation of green tea bioactive compounds | EGCG and caffeine amounted to <19.29 mg/g microbeads and <12.59 mg/g microbeads, respectively | - | (43) |
Table 2. HPLC estimation of green tea catechins.
No. | Mobile phase | Ratio (v/v) | Retention time | Wavelength | References |
1 | Phosphoric acid (0.2%) : CAN : THF | 73.5:25:1.5 | 30 min | 210-280 nm | (44) |
2 | 5% acetonitrile (eluent A) and 25% acetonitrile (eluent B) in phosphate buffer (0.025 M, pH 2.4). | - | - | 278 nm | (45) |
3 | Water : acetonitrile : methanol : ethyl acetate : glacial acetic acid | 8:9:6:1:3:1 | - | 280 nm | (46) |
Table 3. UHPLC estimation of EGCG.
No. | Mobile phase | Ratio (v/v) | Retention time | References |
1 | Phosphate buffer : methanol | 70:30 | 2.14 min | (47) |
2 | Phosphate buffer : ACN | 55:45 | 2.87 min | (48) |
3 | Acetic acid : ACN : water | 13:15:72 | 0.85 min | (49) |
Pharmaceutical Formulations of Green Tea Catechins
Food additives such as vitamin C, xylitol, sucrose, citric acid, butylated hydroxytoluene, dibutyl hydroxytoluene, and ethylenediamine tetraacetic acid have been mixed with green tea extracts and extracted EGCG. Also, they were diluted in milk, soy, and rice beverages, as well as citrus juices like orange, grapefruit, lemon, and lime (39).
Pharmaceutical Analysis of Green Tea Catechins
Several detection methods have been developed for tea catechin analysis, which is largely based on liquid chromatography (LC) methods like High-Pressure Liquid Chromatography, High-Performance Thin Layer Chromatography, and Ultra High-Pressure Liquid Chromatography for getting a good separation, identification, and quantification of the catechins.
HPLC Estimation of Green Tea Catechins
HPLC methods according to previous work has been listed in Table 3.
UHPLC Estimation of Green Tea Catechins
UHPLC methods according to previous work have been listed in Table 4.
HPTLC Estimation of Green Tea Catechins
High-performance thin-layer chromatography (HPTLC) is a simple and extremely sensitive technique that has been shown to identify a variety of phytoconstituents. It also has low solvent consumption and requires a little sample.
According to studies conducted by Thammarat P., et al. (2021). Chromatographic separation was accomplished using silica gel 60 F254-precoated HPTLC plates (20x10 cm). Samples were loaded and developed on the same chromatographic plate. The samples were loaded onto a Camag (Muttenz, Switzerland) Linomat 5 sample applicator equipped with a 100 µl Hamilton syringe. After the plate had been soaked in the developing solvent (toluene: ethyl acetate: acetone: formic acid (6:6:6:1 v/v/v/v)) for 20 min at ambient temperature (25°C), ascending development to a distance of 80 mm was performed. After the plates have dried and been developed, they were measured at 254 nm with a Camag TLC Scanner 4 and WinCAT software.
The developed solvent demonstrated respectable linearity with a correlation coefficient of 0.9951 and reasonable specificity with an Rf value of 0.54 ± 0.02. The recovery was 98.84 % - 103.53 %, and the intra-day and inter-day precision RSDs were, respectively, 0.70 % - 3.00 % and 1.93 % - 4.94 % (50).
According to the study conducted by Reich E., et al. (2006), Chromatographic separation was accomplished using HPTLC plates of silica gel 60 F254. Ethyl formate, toluene, formic acid, and water (30:1.5:4:3) were used as mobile phase. The method was further validated, addressing specificity, stability, reproducibility, and robustness (51).
Conclusion
The most commonly consumed beverage is tea. Due to the strong correlation between drinking tea and having positive health effects, tea polyphenols have drawn a lot of public interest. In this review article pharmacognosy, pharmacological action, phytochemistry, analytical work, and pharmaceutical formulations of green tea catechins have been listed by carrying out a literature review on reported studies. As green tea catechins are believed to improve overall health, we recommend the formulation of a gelatin gummy which will improve its palatability by masking the bitter taste, carry conveniently, and have easy access as compared to green tea. Furthermore, we found the scope to develop an analytical method for EGCG and carry out its validation by HPLC which will be more reliable and cost-efficient as only UHPLC methods exist for EGCG.
Declarations
Ethics Statement
Not applicable.
Data Availability
Not applicable.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Puri V, Nagpal M, Singh I, Singh M, Dhingra GA, Huanbutta K, et al. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients. (2022) 14(21):4637.
- Chaudhari SP. Nutraceuticals: a review. World J Pharm Pharm Sci. (2017) 6(8):681–739.
- Gnk G, A. R, V. S, V. S, Baviya PR. Nutraceuticals - a regulatory review. Int J Drug Regul Aff. (2018) 3(2):22–9.
- Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, Pani L, et al. Nutraceuticals: opening the debate for a regulatory framework: Nutraceutical regulatory framework. Br J Clin Pharmacol. (2018) 4(4):659–72.
- Pandey KB, Rizvi SI. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid Med Cell Longev. (2009) 2(5):270–8.
- Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. (2014) 2:187–95.
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. (2010) 2(12):1231–46.
- Bartosikova L, Necas J. Epigallocatechin gallate: a review. Veterinární Medicína. (2018) 63(10):443–67.
- Aboulwafa, Youssef, Gad, Altyar, Al-Azizi, Ashour. A comprehensive insight on the health benefits and phytoconstituents of Camellia sinensis and recent approaches for its quality control. Antioxidants. (2019) 8(10):455.
- Harima S, Yoshikawa M, Tokuoka K. Historical consideration of tea trees and tea flowers, especially regarding the use of tea flowers as food. Yakushigaku Zasshi. (2008) 43(1):16–32.
- Ekayanti M, Ardiana L, Najib SZ, Sauriasari R, Elya B. Pharmacognostic and Phytochemical Standardization of White Tea Leaf (Camellia sinensis L. Kuntze) Ethanolic Extracts. Pharmacogn J. (2017) 9(2):221–6.
- Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. (2007) (18):3268–95.
- Hwang HJ, Kim YG, Chung MS. Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment. Foods. (2021) 10(12):3092.
- Radovanović K, Gavarić N, Švarc-Gajić J, Brezo-Borjan T, Zlatković B, Lončar B, et al. Subcritical Water Extraction as an Effective Technique for the Isolation of Phenolic Compounds of Achillea Species. Processes. (2022) 11(1):86.
- Fincan M, DeVito F, Dejmek P. Pulsed electric field treatment for solid–liquid extraction of red beetroot pigment. J Food Eng. (2004) 64(3):381–8.
- Mandal R, Mohammadi X, Wiktor A, Singh A, Pratap Singh A. Applications of Pulsed Light Decontamination Technology in Food Processing: An Overview. Appl Sci. (2020) 10(10):3606.
- Namal Senanayake SPJ. Green tea extract: Chemistry, antioxidant properties and food applications – A review. J Funct Foods. (2013) 5(4):1529–41.
- Botten D, Fugallo G, Fraternali F, Molteni C. Structural Properties of Green Tea Catechins. J Phys Chem B. (2015) 119(40):12860–7.
- Balentine DA, Wiseman SA, Bouwens LCM. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. (1997) 37(8):693–704.
- Reygaert WC. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res Int. (2018) 2018:1–9.
- Labidi NS, Guerguer L, Kacemi A. Theoretical Evaluation of Antioxidant Activity of Tea Catechins. J Mater Environ Sci. (2018) 9(1):326–33.
- Ouyang J, Zhu K, Liu Z, Huang J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid Med Cell Longev. (2020) 2020:1–14.
- Epigallocatechin. Available from: https://go.drugbank.com/drugs/DB03823. Accessed June 8 2023.
- Epigallocatechin gallate. Available from: https://go.drugbank.com/drugs/DB12116. Accessed June 8 2023.
- Epicatechin Available from: https://go.drugbank.com/drugs/DB12039. Accessed June 8 2023.
- (-)-Epicatechin gallate. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/107905. Accessed June 8 2023.
- Moore RJ, Jackson KG, Minihane AM. Green tea (Camellia sinensis ) catechins and vascular function. Br J Nutr. (2009) 102(12):1790–802.
- Ullmann U, Haller J, Decourt J, Girault N, Girault J, Richard-Caudron A, et al. A Single Ascending Dose Study of Epigallocatechin Gallate in Healthy Volunteers. J Int Med Res. (2003) 31(2):88–101.
- Bhardwaj P, Khanna D. Green tea catechins: defensive role in cardiovascular disorders. Chin J Nat Med. (2013) 11(4):345–53.
- Oyama J ichi, Maeda T, Sasaki M, Kozuma K, Ochiai R, Tokimitsu I, et al. Green Tea Catechins Improve Human Forearm Vascular Function and Have Potent Anti-Inflammatory and Anti-Apoptotic Effects in Smokers. Intern Med. (2010) 49(23):2553–9.
- Wang ZM, Zhou B, Wang YS, Gong QY, Wang QM, Yan JJ, et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. (2011) 93(3):506–15.
- Tang FY, Meydani M. Green Tea Catechins and Vitamin E Inhibit Angiogenesis of Human Microvascular Endothelial Cells Through Suppression of IL-8 Production. Nutr Cancer. (2001) 41(1–2):119–25.
- Antonello M, Montemurro D, Bolognesi M, Dipascoli M, Piva A, Grego F, et al. Prevention of Hypertension, Cardiovascular Damage and Endothelial Dysfunction with Green Tea Extracts. Am J Hypertens. (2007) 20(12):1321–8.
- Kang WS, Lim IH, Yuk DY, Chung KH, Park JB, Yoo HS, et al. Antithrombotic Activities of Green Tea Catechins and (−)-Epigallocatechin Gallate. Thromb Res. (1999) 96(3):229–37.
- Chen IJ, Liu CY, Chiu JP, Hsu CH. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. (2016) 35(3):592–9.
- Li G, Yang J, Wang X, Zhou C, Zheng X, Lin W. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. (2020) 11(10):8780–7.
- Scholey A, Downey LA, Ciorciari J, Pipingas A, Nolidin K, Finn M, et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite. (2012) 58(2):767–70.
- Bitzer ZT, Elias RJ, Vijay-Kumar M, Lambert JD. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol Nutr Food Res. (2016) 60(10):2267–74.
- Cerbin-Koczorowska M, Waszyk-Nowaczyk M, Bakun P, Goslinski T, Koczorowski T. Current View on Green Tea Catechins Formulations, Their Interactions with Selected Drugs, and Prospective Applications for Various Health Conditions. Appl Sci. (2021) 11(11):4905.
- Aslani A, Ghannadi A, Khalafi Z. Design, formulation and evaluation of green tea chewing gum. Adv Biomed Res. (2014) 3(1):141.
- R.W.N Tanuja, R.P Perera and G.H.C.M.Hettiarachchi. Formulation of green tea polyphenols as solid food supplement. Int J of Adv in pharma res. (2016) 5(1):65–72.
- Moumita S, Das B, Sundaray A, Satpathi S, Thangaraj P, Marimuthu S, et al. Study of soy-fortified green tea curd formulated using potential hypocholesterolemic and hypotensive probiotics isolated from locally made curd. Food Chem. (2018) 268:558–66.
- Belščak-Cvitanović A, Đorđević V, Karlović S, Pavlović V, Komes D, Ježek D, et al. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. (2015) 51:361–74.
- Wangkarn S, Grudpan K, Khanongnuch C, Pattananandecha T, Apichai S, Saenjum C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules. (2021) 26(19):6052.
- Khokhar S, Venema D, Hollman PCH, Dekker M, Jongen W. A RP-HPLC method for the determination of tea catechins. Cancer Lett. (1997) 114(1–2):171–2.
- Saito ST, Welzel A, Suyenaga ES, Bueno F. A method for fast determination of epigallocatechin gallate (EGCG), epicatechin (EC), catechin (C) and caffeine (CAF) in green tea using HPLC. Cienc E Tecnol Aliment. (2006) 26(2):394–400.
- U. V. R, R. SS, Kumar K. R, Narayan Sinha S. Method development and validation for rapid identification of epigallocatechin gallate using ultra-high performance liquid chromatography. Pinheiro M, editor. Plos one. (2020) 15(1):e0227569.
- Ungarala R, Sinha SN, Sunder RS. Ultra high-Performance Liquid Chromatography (UHPLC) method development and validation for the identification of oxidized product of Epigallocatechin-3-Gallate (EGCG). J Chromatogr Sci. (2023) 61(2):140–50.
- El-Kayal MO, Sayed MN, Mortada ND, Elkheshen S. Development and validation of a simple and rapid UPLC method for the in-vitro estimation of (-)-epigallocatechin-3-gallate in lipid-based formulations. Eur J Chem. (2018) 9(1):7–12.
- Thammarat P, Sirilun S, Phongpradist R, Raiwa A, Pandith H, Jiaranaikulwanitch J. Validated HPTLC and antioxidant activities for quality control of catechin in a fermented tea ( Camellia sinensis var. assamica). Food Sci Nutr. (2021) 9(6):3228–39.
- Reich E, Schibli A, Widmer V, Jorns R, Wolfram E, DeBatt A. HPTLC Methods for Identification of Green Tea and Green Tea Extract. J Liq Chromatogr Relat Technol. (2006) 29(14):2141–51.

 ETFLIN
Notification
ETFLIN
Notification