Efficacy of Sunscreen Gel Infused with Giant Tiger Prawn (Penaeus monodon) Head Extract
by Adilah Marwa ★ , Nuraini Ekawati, Hardian Hardian
Academic editor: Garnadi Jafar
Sciences of Pharmacy 2(3): 122-134 (2023); https://doi.org/10.58920/sciphar02030122
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
04 Aug 2023
26 Aug 2023
31 Aug 2023
05 Sep 2023
Abstract: Excessive sunlight exposure can lead to various minor skin disorders, including sunburn and the development of chronic skin malignancies. One effective preventive measure against these adverse effects is the use of sunscreen. Sunscreen can be derived from natural sources, such as the astaxanthin compound in giant tiger prawns (Penaeus monodon). This study aimed to formulate a sunscreen gel from giant tiger prawn head extract that meets good physical gel standards. Additionally, the study sought to determine the sun protection factor (SPF), erythema transmission level (%TE), and pigmentation transmission level (%TP) through in-vitro testing. The extraction process involved using coconut oil as a solvent using the maceration method. The resultant extract was then evaluated for SPF, %TE, and %TP values and subsequently formulated into gel variants with extract concentrations ranging from 1% to 10%. The findings of this investigation revealed that the giant tiger prawn head extract exhibited SPF, %TE, and %TP values of 8.0±0.11, 18.8±0.25%, and 21.7±0.73%, respectively, categorizing it as providing maximum protection, facilitating fast tanning, and acting as a sunblock. The gel formula containing 8% giant tiger prawn head extract demonstrated the highest sunscreen potential. In conclusion, this study highlights the promising potential of giant tiger prawn head extract as a natural sunscreen ingredient and identifies the optimal gel formula for effective sun protection.
Keywords: Giant tiger prawnPenaeus Monodonsunscreen gelerythema transmissionpigmentation transmissionastaxanthinUV protection
Introduction
Sunlight has a vital role in every living creature, one of which is the formation of vitamin D, which is beneficial for bone growth (1). However, exposure to excessive sunlight can cause the epidermal layer of the skin to no longer protect from the harmful effects caused by sun exposure. So, it can cause mild disorders such as sunburn and chronic disorders to skin malignancy. Several ways can be done to prevent those severe effects, one of which is using sunscreen. Sunscreen is a substance that can protect the skin from UV radiation. The effectiveness and activity of sunscreen refer to the value of sun protection factor, percent of erythema transmission, and percent of pigmentation transmission, which reflect the capabilities of sunscreen products in protecting the skin from erythema and pigmentation due to UV exposure (2).
Sunscreen can be obtained from natural ingredients such as astaxanthin compounds in marine life, including giant tiger prawns (Penaeus Monodon) (3). The giant tiger prawn is a marine biota commonly consumed by the public. They contain quite high astaxanthin, namely the head and shell of the prawn (4). However, so far, the prawn heads have been left to rot, and only a few people use them to produce prawn sauce, an Indonesian traditional sauce called “sambal petis,” or prawn crackers. The antioxidant properties of carotenoids (astaxanthin) in the head and shells of giant tiger prawns (Penaeus Monodon) have the potential to be used in various industries, including the cosmetics and pharmaceutical industries (3).
Astaxanthin has a conjugated double-bond structure that inhibits UV exposure to the skin. According to Prasiddha et al., the compounds in sunscreen can protect the skin because of the conjugate bonds, and then these bonds will resonate when exposed to UV rays and reduce their energy so that they can protect the skin from exposure to UV rays (5). Stahl et al. and Hawkins have also studied the protective effect of astaxanthin, where the results show that astaxanthin effectively reduces the formation of polyamine compounds, which can cause skin damage. This study concluded that astaxanthin works by producing enzymes that break down polyamine compounds due to the UV process (6).
The dosage form selection in the sunscreen formula is an important element related to the convenience of use and stability during storage. Gels are semi-solid preparations in which the movement of the dispersing medium is limited by a three-dimensional network of particles or macromolecules dissolved in the dispersing phase. Gel preparations are often used in pharmaceutical, cosmetic, and food products. The selection of this gel form is related to its many advantages: no sticky feeling upon application, ease of washing, and higher spreadability on the skin resulting from the high water content in the gel. The water content in the gel will hydrate the stratum corneum to become more permeable to active substances, increasing the permeation of active substances (7). In addition, in this study, carbopol 940 was used as a gel base because it can form a smooth, transparent gel, and at room temperature, carbopol can maintain its viscosity in the long term (8).
Numerous publications have explored the formulation of sunscreen gel preparations, predominantly sourced from natural plants and chemical synthetics (8, 9, 10). This research introduces an innovative alternative by harnessing astaxanthin derived from tiger prawn heads for sunscreen applications. A pivotal criterion for sunscreen formulations is their ability to efficiently absorb sunlight within the 290-320 nm wavelength range while maintaining stability during use. Thus, this study evaluates the efficacy and stability of sunscreen gel preparations containing tiger prawn head extract.
Materials
and Methods
Materials
The sample material used was the extract of the giant tiger prawn head (Penaeus Monodon) from the ponds in Mororejo Village, Kaliwungu Sub-district, Kendal Regency, Central Java. The giant tiger prawns (Penaeus Monodon) were determined in The Ecology and Systematics Laboratory of the Department of Biology, Faculty of Science and Mathematics, Diponegoro University, Indonesia. Other ingredients used in this study were virgin coconut oil (PT. Okusi Biotech), carbopol 940 (Brataco, Indonesia), triethanolamine (Brataco, Indonesia), propylene glycol (Brataco Indonesia); methylparaben (Brataco, Indonesia); aquadest, hexane (Merck, German); acetone (Merck, German); chloroform (Merck, German); and a muslin cloth (Merck, German).
Giant Tiger Prawn Head Extraction
The sample of prawn heads was separated from the bodies and tails before being washed and added with lime (4). These were then dried in the sun while covered with a black cloth for 4 h. Then, it was dried in the oven at 40oC until dry, then blended and sieved in 80 mesh.
Approximately 28 g of simplicia obtained from giant tiger prawn heads were introduced into coconut oil heated to 70°C and stirred using a magnetic stirrer for 2 h. Subsequently, the resulting extract was centrifugated (Kubota 5100, Japan) at 4500 g per minute for 10 min, maintaining a temperature of 20°C. The supernatant was carefully collected for further analysis, wherein the total astaxanthin content was quantified utilizing a UV-Vis spectrophotometry detector set to a wavelength of 486 nm (Shimadzu, Japan) (12). The yield of astaxanthin (µg/L) was calculated using the equation 1:
Equation 1
A means the absorbance, V implies the volume of oil, D indicates the extinction coefficient of coconut oil (2315), and W means the weight of the sample dissolved in oil.
Astaxanthin Identification using Thin Layer Chromatography
Astaxanthin was identified in shrimp head extract using thin-layer chromatography. The extracted sample was stained on a KLT GF 254 plate with eluent hexane: acetone (3:1) (13). Then, the plate was observed in a spectrophotometer with 254 nm and 366 nm wavelengths. The Rf value was determined using an astaxanthin standard to confirm the presence of astaxanthin.
Sunscreen Gel
Preparation
The sunscreen gel was formulated using varying concentrations of giant tiger prawn head extract, precisely at 1%, 2%, 4%, 6%, 8%, and 10%. Table 1 shows the composition of the sunscreen gel containing giant tiger prawn head extract.
All the ingredients were carefully measured according to the prescribed formula. Methyl and propylparaben were first dissolved in distilled water. Subsequently, carbopol 940 was introduced into the solution and stirred vigorously using a magnetic stirrer, forming a gel mass. Glycerin and TEA were then incorporated into the base and stirred until a homogeneous consistency was achieved. The giant tiger prawn head extract was added to the base gel mixture and stirred until the mixture reached a uniform blend. Once this was accomplished, the gel was transferred into a suitable container, and measurements for SPF, %TE, and %TP were conducted.
Table 1. Sunscreen gel formula.
|
Materials |
Formula |
Roles |
|||||
|
I |
II |
III |
IV |
V |
VI |
||
|
Giant Tiger Prawn Heads |
1% |
2% |
4% |
6% |
8% |
10% |
Sunscreen Active Substance |
|
Carbopol 940 |
2% |
2% |
2% |
2% |
2% |
2% |
Gel Forming Agent |
|
Methyl Paraben |
0,1% |
0,1% |
0,1% |
0,1% |
0,1% |
0,1% |
Preservative |
|
Propyl Paraben |
0,1% |
0,1% |
0,1% |
0,1% |
0,1% |
0,1% |
Preservative |
|
Triethanolamine |
2% |
2% |
2% |
2% |
2% |
2% |
Neutralizer |
|
Propylene glycol |
2% |
2% |
2% |
2% |
2% |
2% |
Humectants |
|
Aquades |
q.s |
q.s |
q.s |
q.s |
q.s |
q.s |
Base |
In Vitro Test
Sun Protection Factor
The astaxanthin extract with a 4% concentration was inserted into the cuvette. Then, a test absorption curve was made with a wavelength of 290 - 320 nm with 5 nm intervals of coconut oil used as a blank. The absorbance results were recorded then the SPF value was calculated using the Mansur method (14).
Table 2. Normalized product function used in SPF calculation.
|
No. |
Wave Length (nm) |
EE × I |
|
1. |
290 |
0.0150 |
|
2. |
295 |
0.0817 |
|
3. |
300 |
0.2874 |
|
4. |
305 |
0.3278 |
|
5. |
310 |
0.1864 |
|
6. |
315 |
0.0839 |
|
7. |
320 |
0.0180 |
|
Total |
1 |
|
The gel from all samples was precisely weighed to 1 g, then transferred into a 100 ml volumetric flask and diluted with chloroform to reach a final volume of 100 ml. Subsequently, ultrasonication was performed for 5 min, followed by filtration through a muslin cloth. The initial 10 ml fraction was discarded, and aliquots of 5 ml each were transferred into separate 50 ml volumetric flasks, then further diluted with chloroform (15). The absorbance of the sample solution was then recorded within the 290 - 320 nm wavelength range, using 5 nm intervals, employing a 1 cm cuvette with chloroform serving as the blank. Using the Mansur method, the recorded absorbance values were then utilized to calculate the SPF value. (16).
Equation 2
Where EE represents the erythema effect spectrum, I represents spectrum intensity, Abs signifies the absorbance of the sunscreen gel, and CF denotes the correction factor, which is fixed at a value of 10. It's important to note that the constants EE and I are predetermined based on the values provided in Table 2 (14).
Erythema Transmission
The test absorption curve of the dissolved gel in the previous stage was made with a wavelength of 292.5 - 337.5 nm with 5 nm intervals. The absorbance results were recorded, and then the calculation was carried out using the Equation 3:
Equation 3
where A means absorbance and T means transmittance. Transmission of erythema (Te) is calculated using the Equation 4:
Equation 4
where means transmission of erythema and Fe means erythema flux. In this case, Fe is the erythema flux whose value is at a particular wavelength (290-320 nm) (16). The amount of erythema flux passed on by sunscreens (Ee) is calculated by Equation 5:
Equation 5
The percentage of erythema transmission (%TE) is calculated using the Equation 6:
Equation 6
Transmission of Pigmentation
The test absorption curve was made with a wavelength of 322.5 - 372.5 nm with 5 nm intervals. The absorption data were recorded, and then the calculation was done using the formula below.
Equation 7
where Tp is transmission of pigmentation, T is transmission, and Fp is flux of pigmentation. The amount of pigmentation flux passed on by the sunscreens (Ep) is calculated by Equation 8.
Equation 8
where Ep means the amount of erythema flux passed on by the sunscreens. % transmission of pigmentation (%TP) is calculated using the following Equation 9.
Equation 9
Formula Characterization and
Cycling Test
The optimization of the gel formula involved a comprehensive series of tests, including organoleptic evaluation, gel dispersion assessment, pH measurement, adhesion analysis, homogeneity examination, and stability assessment. The stability of the gel was scrutinized utilizing the cycling test method, an accelerated approach that involves storing the gel under varying temperature conditions to expedite stability evaluation. The gel was initially stored at a temperature of 40±2°C for a duration of 24 h, followed by placement at 5±2°C for another 24 h. This complete cycle was repeated six times for 12 days (5). The physical stability of the gel was then evaluated based on the organoleptic test, gel dispersion test, pH assessment, adhesion evaluation, and homogeneity analysis.
Data Analysis
The statistical analysis assessed differences in various parameters among sunscreen gel formulations with varying concentrations of giant tiger prawn extract. This included pH values, adhesion, dispersion, SPF values, pigmentation transmission percentage, and erythema transmission percentage. IBM SPSS Statistics 25 was used for data analysis. Levene's Test determined homogeneity, while the Shapiro-Wilk test assessed normality (p<0.05 indicated normal distribution). If the ANOVA test showed significance, the analysis proceeded with the Least Significant Difference (LSD) test. Alternatively, if homogeneity and normality criteria were not met, the Kruskal-Wallis test was employed, followed by the Duncan test if significant differences were observed (p<0.05).
Results
Giant Tiger Prawn Head
Extraction
The astaxanthin yield obtained in this study was 87.05 mg/g, consistent with existing literature that reports astaxanthin content in giant tiger prawns ranging from 10 to 150 mg/kg (18). Detailed results of the giant tiger prawn head extraction can be found in Table 3.
Table 3. Giant tiger prawn head extraction results.
|
Sample Weight |
Simplicia Weight |
The Extracted Simplicial Weight |
Extract Type |
Total Extract |
Total Astaxanthin Yield |
|
4 kg of Giant Tiger Prawn |
250 g |
28 g |
Giant Tiger Prawn Head Oil |
759 ml |
87.05 g/g |
Astaxanthin Identification using
Thin Layer Chromatography
The thin layer chromatography (TLC) analysis results for the astaxanthin derivative revealed distinct stains on the TLC plate, as depicted in Figure 1. Within the sample, a total of eight stains were identified, each corresponding to specific compounds: astaxanthin ester (Rf value 0.10-0.16), trans-astaxanthin (Rf value 0.26), β-cryptoxanthin (Rf value 0.35), canthaxanthin (Rf value 0.40), astaxanthin monoester (Rf value 0.50), and semiastacene (Rf value 0.62-0.67) (12).

Figure 1. The thin layer chromatography under UV light 254 nm (A) and 366 nm (B).
Sun Protection Factor,
Transmission of Erythema, and Transmission of Pigmentation
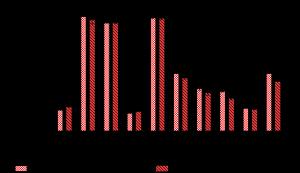
Highest SPF was in giant tiger prawn head extract, similar to FV (8%), FIV (6%), and FIII (4%), with other treatments differing significantly. See Table 4 for average SPF, %TE, and %TP values.
Table 4. Average values of sun protection factor and transmission of erythema and pigmentation.
|
Formula |
Average Value of SPF |
Average Value of %TE |
Average Value of %TP |
|
Extract |
8.0 ± 0.11 |
18.8±0.25 |
21.8±0.73 |
|
Negative Control |
0.005 ± 0.00 |
99.9±0.173 |
97.4±4.16 |
|
Negative Control (10% oil) |
0.2 ± 0.104 |
94.4±0.34 |
94.6±1.02 |
|
Astaxanthin 4% |
7.7 ± 0.24 |
16.0±0.20 |
17.4±0.12 |
|
F I |
0.4 ± 0.027 |
98.9±0.92 |
98.4±1.35 |
|
F II |
2.9 ± 0.089 |
50.5±0.31 |
46.8±1.38 |
|
F III |
4.3 ± 0.13 |
37.5±1.65 |
33.7±2.12 |
|
F IV |
4.4 ± 0.21 |
35.1±0.84 |
28.8±0.95 |
|
F V |
6.7 ± 0.27 |
20.4±0.10 |
19.6±0.65 |
|
F VI |
3.0 ± 0.015 |
50.6±0.32 |
43.7±0.32 |
Physical Properties of Giant
Tiger Prawn Head Sunscreen Gel
The organoleptic assessment of the gel encompassed a visual examination of its texture, color, and odor. Results from the organoleptic test indicated that all gel formulations exhibited favorable organoleptic characteristics. A comprehensive overview of the physical properties evaluation for the giant tiger prawn head extract sunscreen gel is provided in Table 5.
Table 5. Gel characterization evaluation before cycling test.
|
Formula |
Organoleptic |
pH |
Spreadability (cm) |
Adhesivity (s) |
Homogeneity |
|
Negative Control |
Odorless, clear, limpid, and translucent, soft gel |
6 |
5.7±0.52 |
0.8±0.025 |
Homogeneous |
|
Negative Control of Oil 10% |
Odorless, white, limpid, translucent gel with separate oil |
6 |
6.6±0.35 |
0.6±0.046 |
Heterogenous |
|
Positive Control of Astaxanthin 4% |
Odorless, dark red, soft gel |
5 |
5.7±0.45 |
0.5±0.081 |
Homogeneous |
|
F I |
Odorless, white, limpid, slightly translucent, soft gel |
6 |
5.0±0.21 |
0.9±0.081 |
Homogeneous |
|
F II |
Odorless, white bone, not transparent, soft gel |
5 |
5.4±0.38 |
0.8±0.087 |
Homogeneous |
|
F III |
Odorless, white with a slight hint of orange, not transparent, |
5 |
5.7±0.15 |
0.7±0.155 |
Homogeneous |
|
F IV |
Odorless, white with a slight hint of orange, less transparent |
5 |
5.7±0.40 |
0.7±0.030 |
Homogeneous |
|
F V |
It has a slight odor from the active substance, orange, transparent |
5 |
5.9±0.26 |
0.6±0.100 |
Homogeneous |
|
F VI |
It has a slight odor from the active substance, orange transparent, the active substance oil is separated from the base |
7 |
7.0±0.21 |
0.5±0.061 |
Heterogenous |
In the normality and homogeneity tests, all spreadability and adhesivity values for the gel before the cycling test, including three control samples and six different formulas, met the criteria with p-values exceeding 0.001, confirming normal distribution and homogeneity across all concentrations. This allowed for a one-way ANOVA test, which revealed significant differences (p<0.001) among concentrations at the 5% significance level. Subsequently, post hoc LSD tests were conducted to explore individual concentration differences further.
Physical Properties of Gel
After Cycling Test
Physical properties assessment of giant tiger prawn head extracts sunscreen gel after cycling test shown in Table 6.
Table 6. Gel characterization evaluation after cycling test.
|
Formula |
Organoleptic |
pH |
Spreadability (cm) |
Adhesivity (s) |
Homogeneity |
|
Negative control |
Clear, limpid, translucent. |
6 |
4.7±0.21 |
0.7±0.17 |
Homogeneous |
|
Negative control of oil 10% |
Odorless, white, limpid, translucent gel with separate oil. |
6 |
5.8±0.52 |
0.5±0.12 |
Homogeneous |
|
Positive control of astaxanthin 4% |
Basic, dark red. |
5 |
5.5±0.45 |
0.5±0.07 |
Heterogenous |
|
F I |
Odorless, white, limpid, slightly translucent. |
6 |
3.9±0.91 |
0.8±0.015 |
Homogeneous |
|
F II |
Odorless, white bone, not transparent. |
5 |
4.8±0.25 |
0.7±0.015 |
Homogeneous |
|
F III |
Odorless, white with a slight hint of orange, not transparent. |
5 |
4.93±0.21 |
0.6±0.015 |
Homogeneous |
|
F IV |
Odorless, white with a slight hint of orange, less transparent |
5 |
5.3±0.40 |
0.5±0.015 |
Homogeneous |
|
F V |
It has a slight odor from the active substance, carbopol, orange, transparent |
5 |
5.4±0.31 |
0.5±0.021 |
Homogeneous |
|
F VI |
It has a slight odor from the active substance, orange transparent, the active substance oil is separated from the base |
7 |
5.8±0.67 |
0.5±0.026 |
Homogeneous |
Discussion
Sun Protection Factor, Transmission of Erythema, and Transmission of
Pigmentation
Figure 2 reveals the tiger prawn head extract's average SPF value at 8.07±0.11, indicating maximum skin protection for approximately 80 min (19). The extract exhibited erythema transmission of 18.79±0.25%, classifying it as a fast tanning sunscreen, and 21.75% ± 0.73, falling within the sunblock category (19). These findings align with literature categorizing tiger prawn head extract's SPF and erythema transmission as maximum protection and fast tanning (20). The extract's astaxanthin compound, with potent biological antioxidant properties, mitigates hyperpigmentation by absorbing excessive energy from reactive oxygen substances (ROS), including singlet oxygen, preventing melanin formation due to UV radiation and skin damage (21).

Figure 2. Diagram of sun protection factor values of positive (PC) and negative (NC and INC) control groups, tiger prawn head extract (EXC), and the six sunscreen gel formulas (FI-FVI). Values followed by an asterisk (*) are significantly different from negative control and FI groups (NC and INC) (p<0.05).
The SPF tests for the two negative controls yielded shallow average values: 0.004±0.0015 for the control without active substances and 0.22 ± 0.104 for the control with 10% VCO oil. These values significantly differed from the five formulas, confirming that VCO oil has an SPF<1, indicating its lack of effectiveness in sunscreen (22).
Different concentrations of tiger prawn head extract resulted in varying SPF values. Formula I exhibited an average SPF value of 0.43 ± 0.24, indicating no sun protection. Formula II had an average SPF of 2.91 ± 0.089, providing minimal protection for 30 min. Formulas III and IV achieved SPF values of 4.29 ± 0.13 and 4.38 ± 0.21, classifying them as moderate protection for 43 and 44 min, respectively. Formula V, with 8% tiger prawn head extract, had an SPF of 6.7 ± 0.27, offering extended protection for 67 min (19). Conversely, Formula VI exhibited a significantly lower SPF of 3.05 ± 0.015, providing minimal protection for only 30 min due to gel separation caused by high extract concentration (23). The positive control gel containing 4% pure astaxanthin achieved an SPF of 7.74 ± 0.24, offering extra protection for 78 min. Notably, this SPF differed significantly from Formula III with the same 4% concentration of tiger prawn head extract, which had a lower SPF.
The measurement results revealed remarkably high %TE and %TP values for the two negative controls: 99.89 ± 0.173% and 97.46 ± 4.16% for controls without active substances and 94.43 ± 0.34% and 94.59 ± 1.02% for controls with 10% VCO oil (see Figure 3). These percentage values for erythema transmission and pigmentation were significantly distinct from those of the five formulas (p>0.05).

Figure 3. Diagram of %erythema transmission and %pigmentation transmission values of positive (PC) and negative (NC and INC) control groups, tiger prawn head extract (EXC), and the six sunscreen gel formulas (FI-FVI). Values are expressed as mean values ± (n = 3). Values followed by an asterisk (*) are significantly different from negative control (NC and INC) and FI groups (p<0.05).
Figure 3 shows the impact of varying the tiger prawn head extract level on erythema transmission percentage values. In Formula I, the mean %TE and %TP values were 98.95 ± 0.92% and 98.45 ± 1.3, indicating its inability to prevent skin erythema and pigmentation (24). Formula II exhibited mean %TE and %TP values of 50.49 ± 0.31 and 46.82 ± 1.38, categorizing it as a fast tanning sunscreen, which darkens the skin rapidly and allows full transmission of UV A radiation (25). Formula III recorded mean %TE and %TP values of 37.46 ± 1.65 and 33.74 ± 2.12, while Formula IV had values of 35.15 ± 0.84 and 19.65 ± 0.91. Formulas III and IV fall into the pigmentation sunblock category, providing full pigmentation protection but not erythema prevention (21).
Formula V, with 8% tiger prawn head extract, displayed mean %TE and %TP values of 20.45 ± 0.10 and 19.65 ± 0.65, indicating its ability to prevent erythema and categorizing it as a sunblock offering maximum protection from erythema and pigmentation. Conversely, Formula VI recorded significantly increased mean %TE and %TP values of 50.64 ± 0.59 and 43.72 ± 0.32, failing to qualify as adequate erythema protection due to the gel separation at 10% concentration, resulting in substantial erythema transmission (lack of UV protection).
Comparatively, the average %TE value for the positive control, containing 4% pure astaxanthin, was 16.09 ± 0.20, classifying it as fast tanning. Meanwhile, the average %TP for the gel containing 4% tiger prawn head extract was 37.46 ± 1.65, falling short of the erythema protection requirements against UV rays. The higher erythema transmission percentage in the extract gel with the same concentration as the positive control could be attributed to the lower concentration of pure astaxanthin in the extract (26).
Physical Properties of Giant Tiger Prawn Head Sunscreen Gel
The results of the spreadability test on the positive control gel, negative control, negative oil control, FI, FII, FIII, FIV, FV, and FVI met the criteria for a good spreadability test of 5-7 cm (27). The pH test results on the FI, FII, FIII, FIV, and FV gels decreased from pH 6 to pH 5. This is in line with the literature, stating that the increase in VCO concentration is in line with the increase in the content of fatty acids. The greater the number of fatty acids, the greater the amount of H+ that dissociated. It caused the pH of the gel to decrease. Whereas in the gel containing 10% giant tiger prawn head extract at pH 7, there was an increase in pH because the gel base and the active substance of giant tiger prawn head extract were separated, leading to a significant increase in pH (28). This shows that a gel with a concentration of 10% giant tiger prawn head extract is unsafe because if a sunscreen has an over-alkaline pH, it will cause scaly skin (29).
The results of the gel adhesion test for positive control, negative control, and negative control for oil, FI, FII, FIII, FIV, FV, and FVI showed to have an adhesive power range of 50-90 s so that all gel formulas met the adhesion test criteria, where the good adhesion of topical formulation should be more than 4 s (29). The results of this study also showed that increasing the concentration of the extract reduced the sticking time.
Homogeneity test results for the positive control gel, negative control, FI, FII, FIII, FIV, and FV showed that they were homogeneous and had no coarse granules. Meanwhile, the negative control gel containing 10% oil and the one containing 10% giant tiger prawn head extracted gel was not homogeneous, and there were oil droplets. This aligns with the literature, which states that the higher the VCO concentration added, the more droplets formed (29).
Physical Properties of Gel After Cycling Test
This cycling test was carried out by storing samples of the nine gel formulas at two different temperatures for six cycles, where each cycle consisted of a low-temperature storage of 5±2oC for 24 h and a high-temperature one of 40±2oC for 24 h. This test aimed to accelerate changes usually occurring under normal conditions (30).
Organoleptic test results on positive, negative, and negative control oil, FI, FII, FIII, and FIV, showed no color, odor, or texture change up to six cycles. Meanwhile, in FV and FVI, changes in shape and color occurred, where the color became more orange, and the smell of the active substance was more dominant. The factor that influenced the color change was the browning reaction (browning). The browning reaction occurs due to the acceleration of oxidation that occurs during storage (8). Besides, the factor that makes the gel and the active substance separate was due to storage at high temperatures. The high temperature will increase the distance between particles so that the force between particles will decrease. The greater distance causes the viscosity to decrease so that the base and the active substance can separate (31). The spreadability test results on positive control, negative control, negative oil control, FI, FII, FIII, FIV, FV, and FVI showed that the cycling test caused a significant reduction in the spreadability. This was due to changes in resistance that occurred in the gel, so the gel's consistency changed (29).
The pH test results on the FI, FII, FIII, FIV, and FVI gels did not change significantly before and after the cycling test. There was an increase in pH in the FV gel because, after the cycling test, the gel on FV underwent physical changes. The base and a little of the active substance were separated.
The results of the adhesivity assessment on the gel, including the positive control, negative control, negative control oil, FI, FII, FIII, FIV, FV, and FVI, showed that the adhesive power range from 40 to 80 s, indicating that all gel formulas after the cycling test, met the criterion of the test. The homogeneity test results after the cycling test on positive control gel, negative control, FI, FII, FIII, FIV, and FV did not show any changes, as the gel remained homogeneous, and there were no coarse grains. Meanwhile, the negative control gel with 10% oil and the one with 10% giant tiger prawn head extract gel showed a change, as the gel became homogeneous. This happened because the negative control gel with 10% oil and the one with 10% giant tiger prawn head extract gel after the cycling test showed a phase separation between the base and the active substance of prawn head oil so that the oil droplets in the gel reduced.
Conclusion
The SPF, %TE, and %TP values of giant tiger prawn head extract (Penaeus monodon) were 8.0±0.11, 18.8±0.25%, and 21.7±0.73%, categorizing it as providing maximum protection, fast tanning, and sunblock. Increasing the concentration of giant tiger prawn head extract impacted the SPF value, %TE, %TP, and the physical stability of the gel. As the extract concentration increased, the sunscreen potential of the gel improved, albeit at the expense of reduced physical consistency. In conclusion, the sunscreen gel formulated with an 8% concentration of giant tiger prawn head extract exhibited the highest sunscreen efficacy.
Declarations
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Mead MN. Benefits of sunlight: a bright spot for human health. Environ Health Perspect. 2008;116(4).
- Geoffrey K, Mwangi AN, Maru SM. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharm J. 2019;27(7):1009–18.
- Viera I, Pérez-Gálvez A, Roca M. Bioaccessibility of marine carotenoids. Mar Drugs. 2018;16(10):1–21.
- Handayani AD, Sutrisno, Indraswati N, Ismadji S. Extraction of astaxanthin from giant tiger (Panaeus monodon) shrimp waste using palm oil: Studies of extraction kinetics and thermodynamic. Bioresour Technol. 2008;99(10):4414–9.
- Prasiddha IJ, Laeliocattleya RA, Estiasih T. Potensi senyawa bioaktif rambut jagung (zea mays L) untuk tabir surya alami : Kajian Pustaka. J Pangan dan Agroindustri. 2016;4(1):40–5.
- Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71(3):795–8.
- Bianchini JM, Zhang Q, Hanna G, Flach CR, Wang H, Southall MD, et al. A unique gel matrix moisturizer delivers deep hydration resulting in significant clinical improvement in radiance and texture. Clin Cosmet Investig Dermatol. 2019;12:229–39.
- Supapvanich S, Mitrsang P, Srinorkham P, Boonyaritthongchai P, Wongs-Aree C. Effects of fresh Aloe vera gel coating on browning alleviation of fresh cut wax apple (Syzygium samarangenese) fruit cv. Taaptimjaan. J Food Sci Technol. 2016;53(6):2844–50.
- Wulansari D, Aripudin, Ratnaningtyas S, Gopurullah SS. Formulation and Physical Evaluation of Sunscreen Cream with Methanol Extract of Euchema cottonii. Nat Sci J Sci Technol. 2022;11(02):37–41.
- Sander M, Sander M, Burbidge T, Beecker J. The efficacy and safety of sunscreen use for the prevention of skin cancer. Cmaj. 2020;192(50):E1802–8.
- Lourith N, Kanlayavattanakul M, Chingunpitak J. Development of sunscreen products containing passion fruit seed extract. Brazilian J Pharm Sci. 2017;53(1):1–8.
- Waste C, Solvents O, Dalei J, Biologicals N, Honda BF, Roads NX, et al. Extraction and Characterization of Astaxanthin From the Crustacean. Int J Pharm Sci Res. 2015;6(6):2532–7.
- Najafi N, Ahmadi AR, Hosseini R, Golkhoo S. Gamma irradiation as a useful tool for the isolation of astaxanthin-overproducing mutant strains of Phaffia rhodozyma. Can J Microbiol. 2011;57(9):730–4.
- Mali SS, Killedar SG. Formulation and in vitro evaluation of gel for SPF determination and free radical scavenging activity of turpentine and lavender oil. Pharma Innov J. 2018;7(3):85–90.
- Patil S, Patil V, Ghodke D, Kondawar M, Naikwade N, Magdum C. Formulation of Gel and Its UV Protective Study of Some Medicinal Flowers. Asian J Pharm Ana. 2011;1(2):34–5.
- Kusmita L, Nur Prasetyo Edi A, Dwi Franyoto Y, Mutmainah, Haryanti S, Dwi Retno Nurcahyanti A. Sun protection and antibacterial activities of carotenoids from the soft coral Sinularia sp. symbiotic bacteria from Panjang Island, North Java Sea. Saudi Pharm J. 2023 Aug;31(8):101680.
- Mishra A, Mishra A, Chattopadhyay P. Assessment of in vitro sun protection factor of Calendula officinalis L. (asteraceae) essential oil formulation. J Young Pharm. 2012;4(1):17–21.
- Angell A, de Nys R, Mangott A, Vucko MJ. The effects of concentration and supplementation time of natural and synthetic sources of astaxanthin on the colouration of the prawn Penaeus monodon. Algal Res. 2018;35(July):577–85.
- USFDA. Employee Health and Personal Hygiene Handbook. FDA Centen. 2020;(1).
- Latha MS, Martis J, Shobha V, Shinde RS, Bangera S, Krishnankutty B, et al. Sunscreening agents: A review. J Clin Aesthet Dermatol. 2013;6(1):16–26.
- Liao WL, Nur-E-Borhan SA, Okada S, Matsui T, Yamaguchi K. Pigmentation of Cultured Black Tiger Prawn by Feeding with a Spirulina-Supplemented Diet. Nippon Suisan Gakkaishi. 1993;59(1):165–9.
- Gause S, Chauhan A. UV-blocking potential of oils and juices. Int J Cosmet Sci. 2016;38(4):354–63.
- Yaghoubi A, Ghojazadeh M, Abolhasani S, Alikhah H, Khaki-Khatibi F. Correlation of Serum Levels of Vitronectin, Malondialdehyde and Hs-CRP With Disease Severity in Coronary Artery Disease. J Cardiovasc Thorac Res. 2015;7(3):113–7.
- Slim Smaoui. Cosmetic emulsion from virgin olive oil: Formulation and bio-physical evaluation. African J Biotechnol. 2012;11(40):9664–71.
- Brenner M, Hearing JV. The protective role of melanin against UV. Photochem Photobiol. 2008;84(3):539–49.
- Ambati RR, Moi PS, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications - A review. Mar Drugs. 2014;12(1):128–52.
- Pratama G, Yanuarti R, Ilhamdy AF, Suhana MP. Formulation of sunscreen cream from Eucheuma cottonii and Kaempferia galanga (zingiberaceae). IOP Conf Ser Earth Environ Sci. 2019;278(1).
- Hernanto M, Suswardana, Saraswati PDA, Radiono S. Virgin Coconut Oil Protection Against UVB Induced Erythema and Pigmentation. Univ Gadjah Mada. 2008;208–11.
- Curnow A, Owen SJ. An Evaluation of Root Phytochemicals Derived from Althea officinalis (Marshmallow) and Astragalus membranaceus as Potential Natural Components of UV Protecting Dermatological Formulations. Oxid Med Cell Longev. 2016;2016.
- Yanti Eff AR, Rahayu ST, Saraswati H, Munim A. Formulation and Evaluation of Sunscreen Gels Containing Mangiferin Isolated from Phaleria macrocarpa Fruits. Int J Pharm Investig. 2019;9(3):141–5.
- Paul SP. Ensuring the Safety of Sunscreens, and Their Efficacy in Preventing Skin Cancers: Challenges and Controversies for Clinicians, Formulators, and Regulators. Front Med. 2019;6(September):1–7.

 ETFLIN
Notification
ETFLIN
Notification