In Silico Larvicidal Activity Study of Six Limonoids Against Mosquito Larvae (Aedes aegypti L.) Ecdysone Receptor Protein
by Mohamed Said Rajab ★
Academic editor: Mohd Shahezwan Abd Wahab
Sciences of Phytochemistry 3(1): 20-26 (2024); https://doi.org/10.58920/sciphy0301217
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
30 Jan 2024
12 Mar 2024
14 Mar 2024
15 Mar 2024
Abstract: In an earlier study, six limonoids namely pyroangolensolide, calodendrolide, limonin, limonin diosphenol, harrisonin and pedonin were reported to exhibit varying larvicidal activity against Aedes aegypti L. second instar larvae. The degraded limonoids exhibited a higher larvicidal activity relative to the more complex compounds. To investigate this observation at the relevant Aedes aegypti L. receptor level, the six limonoids were subjected to an in silico docking study to evaluate the binding characteristics of the selected limonoids in the ecdysone receptor (EcR) protein (PDB code 1z5x). This was compared with the binding affinity of the dipteran specific ecdysone agonist, RH 5849 (1,2-Dibenzoyl-1-tert-butylhydrazine). The EcR protein1z5x-LBP was identified from literature data. The binding energies of the ligands docked in the EcR protein 1z5x-LBP ranged from 3.0 to -9.1 kcal/mol and the dissociation constants (Kd) ranged from 2.10×10-7 M to 1.59×10+2 M. RH 5849 had a binding energy of -8.9 kcal/mol which was comparable with those displayed by pyroangolensolide (-9.1 kcal/mol) and calodendrolide (-9.0 kcal mol). Two pharmacophoric factors were important in the observed binding: (a) the hydrogen-bonding interactions by the residues Arg 271, Arg 275 Tyr 296. Thr231 and Ala 286 and (b) the hydrophobic pocket residues Met 268, Met 272, Met 269, Phe 285, and Leu 308. The binding affinities of the selected limonoids in the EcR pocket compared well with the observed larvicidal activity as reported earlier and in the literature. This study offers an opportunity to develop structurally simpler and specific receptor targeted larvicides against Aedes aegypti L.
Keywords: LimonoidsReceptor protein 1z5xMolecular dockingLarvicidal activity
Introduction
The vector, Aedes aegypti L. is known for transmitting viruses such as yellow fever, chikungunya, dengue, zika and multiple other animal diseases (1). In view of the global warming calamity, it has become apparent that the risk of mosquito-transmitted diseases in a wetter, warmer and highly populated poor areas of the world is bound to increase (2, 3). A number of anti-mosquito insecticides currently in use present disadvantages such as toxicity to humans and other non-target species, degradation of the aquatic environment, high annual costs, and the development of target-resistant population (4). To mitigate these challenges, efficient and cost effective approaches are required to identify innovative mosquito control strategies in addition to developing new insecticides that overcome the adverse problems associated with insecticides currently in use (5). Efforts by researchers to develop new environmentally friendly insecticidal compounds against mosquitos, have yielded various compounds both synthetic and naturally occurring with potential larvicidal activity against various mosquito species (6-9). In silico studies augmented with experimentally generated data is increasingly becoming a tool of choice in the search of new target specific and environmentally friendly insecticidal compounds (10-15).
In the present work, the limonoids, pyroangolensolide synthesized by reducted calodendrolide (pyroangolensolide), calodendrolide, limonin, and limonin diosphenol from Calodendrum capensi, while harrisonin and pedonin from Harrisonia abyssinica (Figure 1) reported earlier as potential mosquito larvicides were subjected to an in silico study involving the docking of the six limonoids (1-6) in the Ecdysone Receptor (EcR) protein 1z5x-Ligand Binding Pocket (LBP) (16, 17). This was compared with the binding affinity displayed by the dipteran specific ecdysone agonist, RH 5849 (1,2-Dibenzoyl-1-tert-butylhydrazine). The EcR protein1z5x-LBP was identified from literature data using the binding characteristics of the ecdysteroid ponasterone A in the receptor pocket (18, 19). It is important to note that the EcR protein is absent in mammals and is therefore a useful receptor target in the development of environmentally safe insecticides which are insect specific and effective by regulating the larva moulting, metamorphosis and reproduction (20). Recent literature shows that generally insecticide potency and selectivity is derived from differential packing of residues within the walls of the ecdysteroid receptor protein binding pockets (18-20).
In order to establish which hydrogen bonds are most important in these interactions, molecular docking was performed using the selected limonoids as the ligands and protein 1z5x as the receptor protein. It is anticipated that the results obtained will lead to a better understanding of the binding site interactions of the selected limonoids, the EcR inhibition characteristics and the limonoid structural features necessary for larvicidal activity. Based on ligand-receptor interactions, molecular docking can be applied as a useful and affordable tool for insecticide discovery.
The present study corroborates earlier studies in the literature and demonstrates the potential of in silico approaches in understanding the modes of action of the limonoids as larvicides against Aedes aegypti L. This will offer opportunities for the development of novel, target specific and safe insecticides.
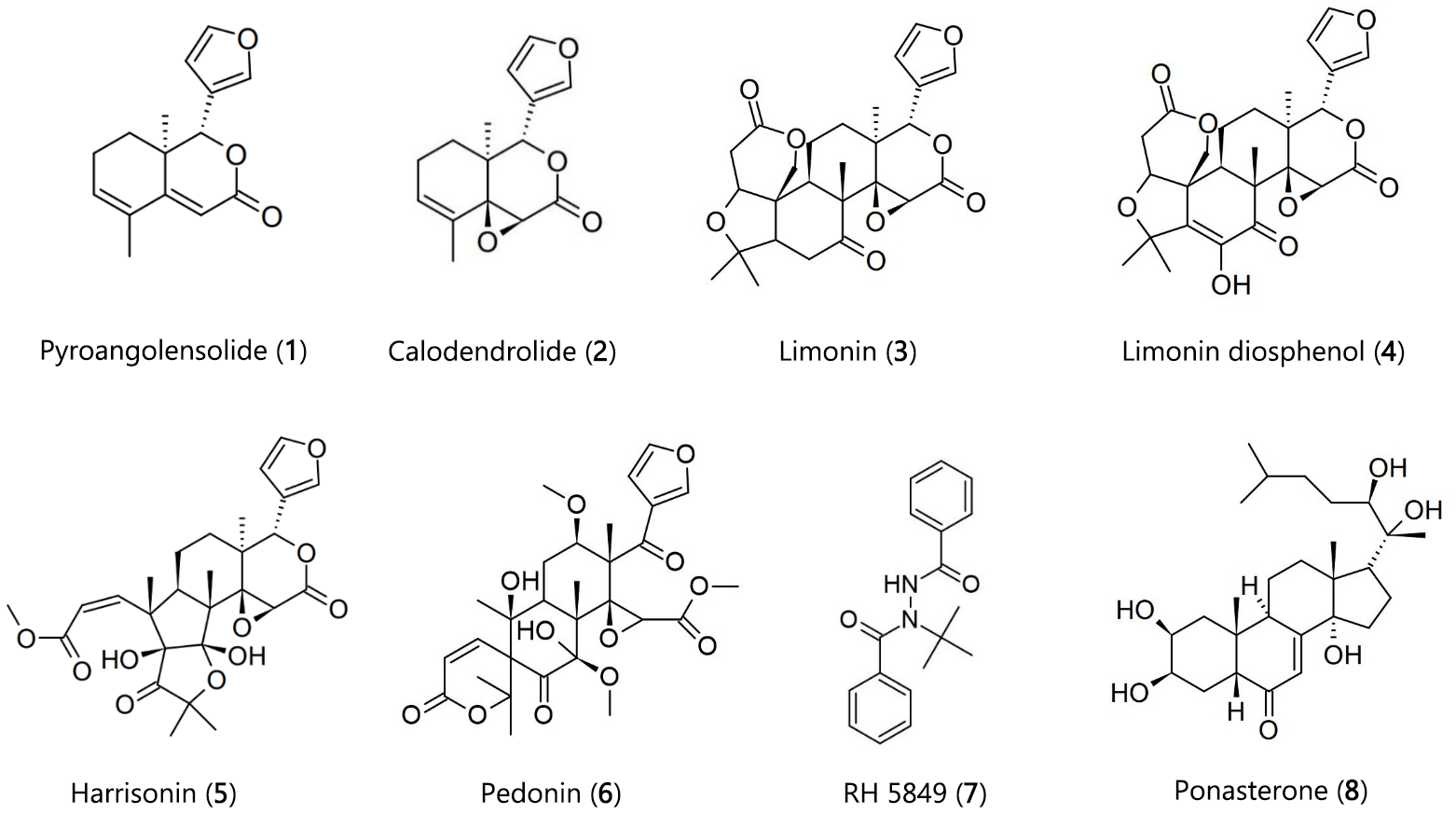
Figure 1. 2D structures of selected limonoids, RH 5849, and ponasterone A.
Experimental Section
Molecular docking was carried out using Autodock Vina imbedded in PyRx 0.8. The protein 1z5x was employed as the receptor protein (18). The selected limonoids were used as ligands. The known insecticide RH 5849 was used as a reference ligand. The ecdysteroid ponasterone A co-crystallized with the EcR protein 1z5x was used to identify the EcR-LBP and also to validate the docking exercise. The docking grid center was placed in the determined EcR-LBP.
Ligand Retrieval and Preparation
Six limonoids (Figure 1) whose larvicidal potential was investigated in vivo in an earlier study were selected for the current in silico study (16, 17). In addition, RH 5849 a diptera specific insecticide was used as a comparative standard (21). The ecdysteroid ponasterone A, was used to identify the LBP and to validate the docking exercise (18, 19, 22). 3D structures of compounds to be studied were downloaded in .sdf format from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The compounds were then energy minimized by Open Babel 2.3.1 using the mmff94 force field with an energy gradient of 0.05 using the steepest descent algorithm, 500 numbers steps, and 10e-7 convergence parameter to generate an energetically reasonable posture for docking. The various optimized 3D structures were finalized for docking using Autodock tools 1.5.7 and saved as .pdbqt files for the subsequent steps.
Receptor Preparation and Molecular Docking
The 3D structure of the EcR protein 1z5x was obtained from the Protein Data Bank repository. The receptor protein was prepared for docking using UCSF ChimeraX, (Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases). The preparation involved removing the already-bound heteroatoms, addition of hydrogens, and addition of charges. Protein–ligand docking was performed in AutoDock tools 1.5.7 (Scripps Research, La Jolla, California, USA).
Docking studies for the target protein (1z5x) and the various ligands (limonoids) were performed using Auto Dock Vina embedded in PyRx 0.8 and visualized using Pymol, Protein Plus and Discovery Studio Visualizer version 2020. The grid box was made to encompass the receptor pocket identified by the proteins binding to the known ecdysteroid ponasterone A. The vina search space parameters used were: center x= 22.230, center y= 67.091, center z= 3.224, size x= 20.546, size y= 21.243, size z=26.243 in Angstrom and exhaustiveness of 8. The analysis and visualization of the binding interaction of protein and ligands were performed using Discovery Studio Visualizer v 4.0. The docking results were clustered on the basis of root mean square deviation (RMSD) and ranked on the basis of free energy of binding. The binding postures of the various ligands were analysed using Ligplot plus ver 2.2.8 Protein Plus and the Discovery Visualizer v 4.0. The dissociation constants Kd (M) was calculated by the online tool NovoPro at www.novoprolabs.com/tools/deltag2kd.
Prediction of Insecticide Potency (Tice Rule)
Bioavailability, bioactivity, and toxicity of compounds are considered as important parameters for development of insecticides. The insecticide-likeness of the limonoids was predicted using Tice rule. According to Tice rule, insecticidal compounds should have: (a) molecular weight <500 g/mol, (b) number of hydrogen-bond donors <3, (c) number of hydrogen-bond acceptors <12, (d) partition coefficient (log P) <5 and (e) no. of rotatable bonds <12 (23). The molecular properties of the selected limonoids were computed by using the online Molinspiration Cheminformatics free web services, https://www.molinspiration.com, Slovensky Grob, Slovakia (www. molinspiration.com) and MedChem Designer(TM) Ver 5.5.0.11.
Docking Validation
Redocking of ponasterone A, a co-crystallized ligand was performed. The bound Ponasterone A, found in the crystal structure was extracted and docked into the corresponding binding pocket. The ability of the ligand to reproduce the orientation and the position of the ligand in the bound form were determined. The RMSD of the result was determined to be 0.7476 A˚ (using Discovery Studio), which suggests the reliability of the molecular docking procedure. The interactions with the amino acids in the receptor pocket are shown in Figure 2.
Results and discussion
Molecular Docking
In the present study, six limonoids with larvicidal potential were subjected to in silico molecular docking on the ecdysone receptor protein 1z5x-Lignd Binding Domain (LBD). The LBD is usually involved in receptor dimerization, ligand recognition and cofactor protein interactions (24). Apart from these, the LBD also contains the ligand-binding pocket (LBP), which binds ecdysteroids as well as other EcR agonists (25-27). A docking, study was performed to evaluate the binding potential of the selected six limonoids to the EcR-LBP this was compared with the commercial insecticide RH 5849. The order of the binding based on docking score is pyroangolensolide (-9.1 kcal/mol), calodendrolide (-9.0 kcal/mol), limonin (-6.8 kcal/mol), harrisonin (-5.6 kcal/mol), limonin diosphenol (-2.3 kcal/mol), pedonin (3.0 kcal/mol) and RH 5849 (-8.9 kcal/mol) (see Table 1). Both pyroangolensolide and calodendrolide had comparable docking scores and were both bound to the binding site by a conventional H-bond with the same amino acids (Arg 271 and Ala 286). Also, both displayed a carbon H-bond interaction with Thr 234, alkyl and pi alkyl interaction with Arg 275, Met 268 and Met 272 (Table 1, Figure 3). Limonin was bound with one conventional H-bond interaction with Arg 275, alkyl and pi alkyl interaction with Met 272, Phe 285, Met 268, Met 269 and Leu 308. The remaining three limonoids also displayed binding within the EcR protein 1z5x-LBP but all the three had at least one unfavourable bump implying unfavourable steric factors at the LBP as shown in Figure 3. The best docked limonoids were selected based on the binding energy and good interaction with the active site’s amino acid residues. The binding energies and the various ligand-receptor amino acids interactions are listed in Table 1.
The docking results with the ecdysteroid ponasterone A (8) and the docking with the six limonoids and RH 5849 showed that Glu199, Arg271, Met 268, Met 272, Phe 285, IIle 227, Tyr 296, Thr 234, Thr 231, Ala286 make up the LBP of the EcR protein 1z5x-LBP. The selected limonoids generally bind to the core of the EcR protein 1z5x-LBD, which consist of hydrophobic amino acids (Ile, Phe and Met), lipophobic amino acids (Thr and Tyr) and hydrophilic amino acids (Thr, Arg and Glu). The electrostatic bond and van der Waals interactions within EcR protein 1z5x-LBP and the ligands are presented in Table 1, Figure 3, and Figure 4. Superimposed graphical representation of the six docked limonoids with EcR protein 1z5x-LBP is shown in Figure 2. The dissociation constants Kd is a measure of binding affinity which is used to evaluate and rank the order of strengths of bimolecular interactions of the ligand and the target protein. The smaller the Kd value, the greater the binding affinity of the ligand to the receptor protein.
Table 1. Interactions of EcR protein 1z5x-LBP residues with selected limonoids and the ecdysone agonist RH 5849.
|
Complex Ligand |
Binding Energy (kcal/mol) |
Predicted dissociation constant Kd |
Number of the various interactions |
||||
|
Conventional H-Bonds |
Alkyl |
pi-interactions |
Carbon -Hydrogen |
Steric clashes |
|||
|
Limonin |
-6.8 |
1.02×10-5 |
1 |
0 |
5 |
0 |
0 |
|
Calodendrolide |
-9.0 |
2.49×10-7 |
2 |
2 |
1 |
1 |
0 |
|
Limonin diosphenol |
-2.3 |
2.05×10-2 |
1 |
0 |
4 |
1 |
3 |
|
Harrisonin |
-5.6 |
7.77×10-5 |
2 |
0 |
2 |
2 |
1 |
|
Pedonin |
3.0 |
0 |
0 |
2 |
2 |
3 |
|
|
Pyroangolensolide |
-9.1 |
2.10×10-7 |
2 |
1 |
1 |
1 |
0 |
|
RH 5849 |
-8.9 |
2.94×10-7 |
1 |
1 |
7 |
0 |
0 |
Table 2. Tice rule properties of scored limonoid poses.
|
Ligand |
Mwt |
LogP |
Rule of 5 violations |
No. of rotatable bonds |
H-bond acceptor (O + N) |
T PSA |
H-bond donor (OH +NH) |
|
Limonin |
470.523 |
2.53 |
0 |
1 |
8 |
104.57 |
0 |
|
Calodendrolide |
260.292 |
2.78 |
0 |
1 |
4 |
51.97 |
0 |
|
Limonin diosphenol |
484.506 |
2.01 |
0 |
1 |
9 |
124.8 |
1 |
|
Harrisonin |
516.549 |
1.98 |
1 (Mw) |
4 |
10 |
145.03 |
2 |
|
Pedonin |
576.602 |
1.21 |
2 (Mw, NO) |
6 |
12 |
171.33 |
2 |
|
Pyroangolensolide |
244.292 |
3.34 |
0 |
1 |
3 |
39.44 |
0 |
Tice Rule and Potency of Ecdysteroid Agonists
No violation of Tice rule was observed in limonin, calodendrolide, limonin diosphenol, pyroangolensolide and ponasterone A, where as harrisonin and pedonin having Mwt > 500 g/mol violated the requirement of molecular weight (23). In addition, pedonin also violated Tice rule on hydrogen bond acceptor requirement (Table 2). Based on this, it can be presumed that the strong larvicidal activity of calodendrolide and pyroangolensolide may be due to their interaction with EcR-1z5x-LBP through their ecdysteroid agonist activity. Further, in-depth laboratory and field studies are needed to support this claim.
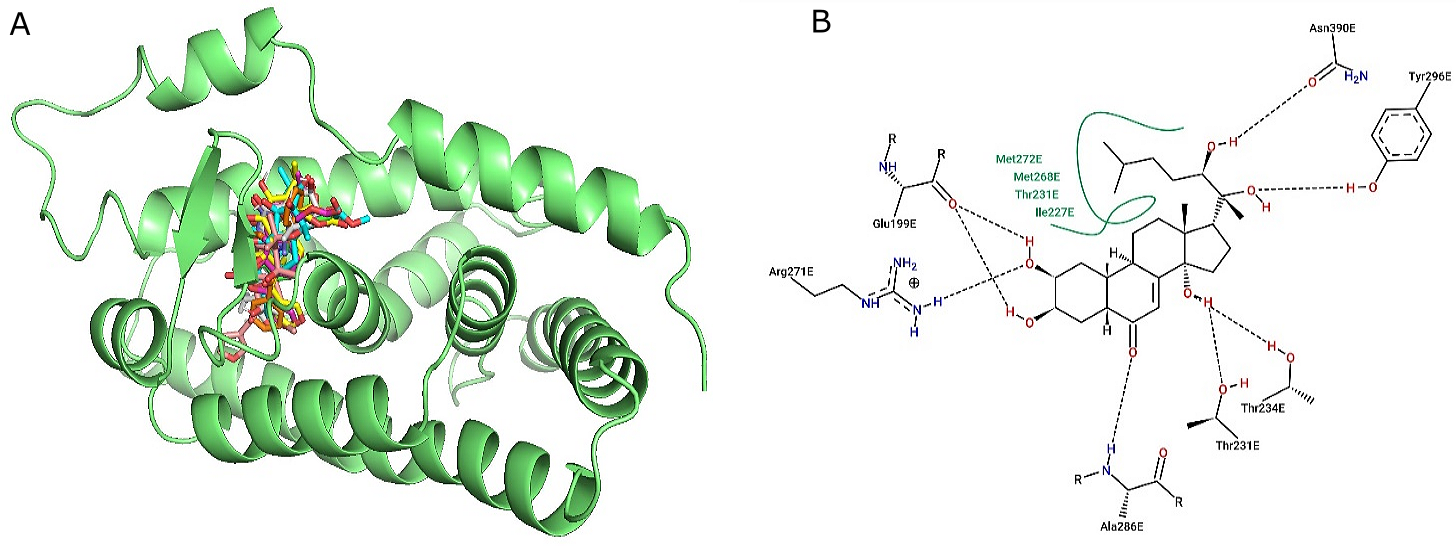
Figure 2. The ecdysone receptor pocket of the target protein in (A) 3D format docked with the the six limonoids, RH5849, and ponasterone A, and (B) 2D structure showing interactions of the relevant amino acids associated with the receptor pocket of the protein 1z5x with ponasterone A.
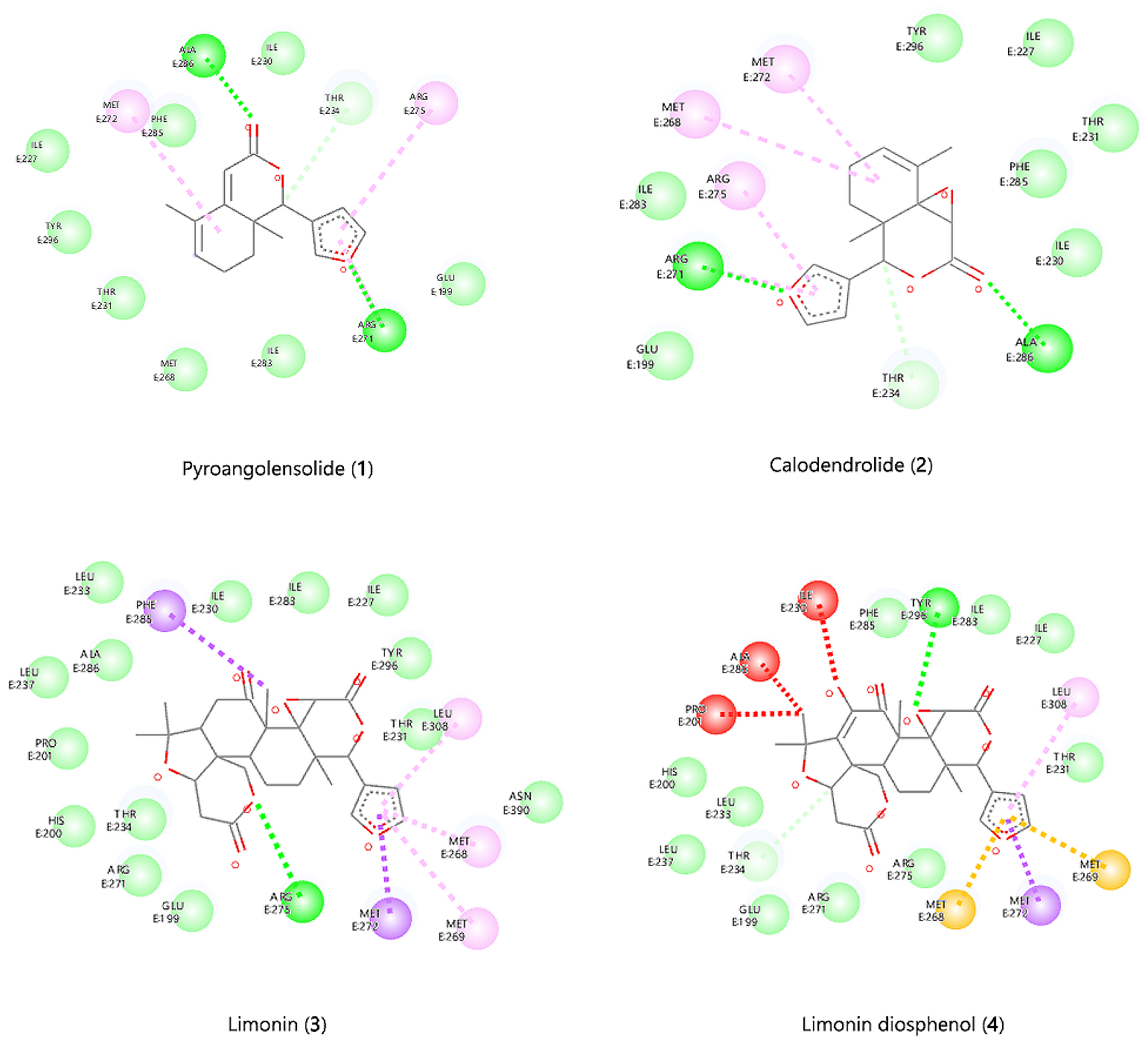
Figure 3. 2D diagrams showing interactions of amino acids in the receptor pocket docked with the most favourable pose of the limonoids.
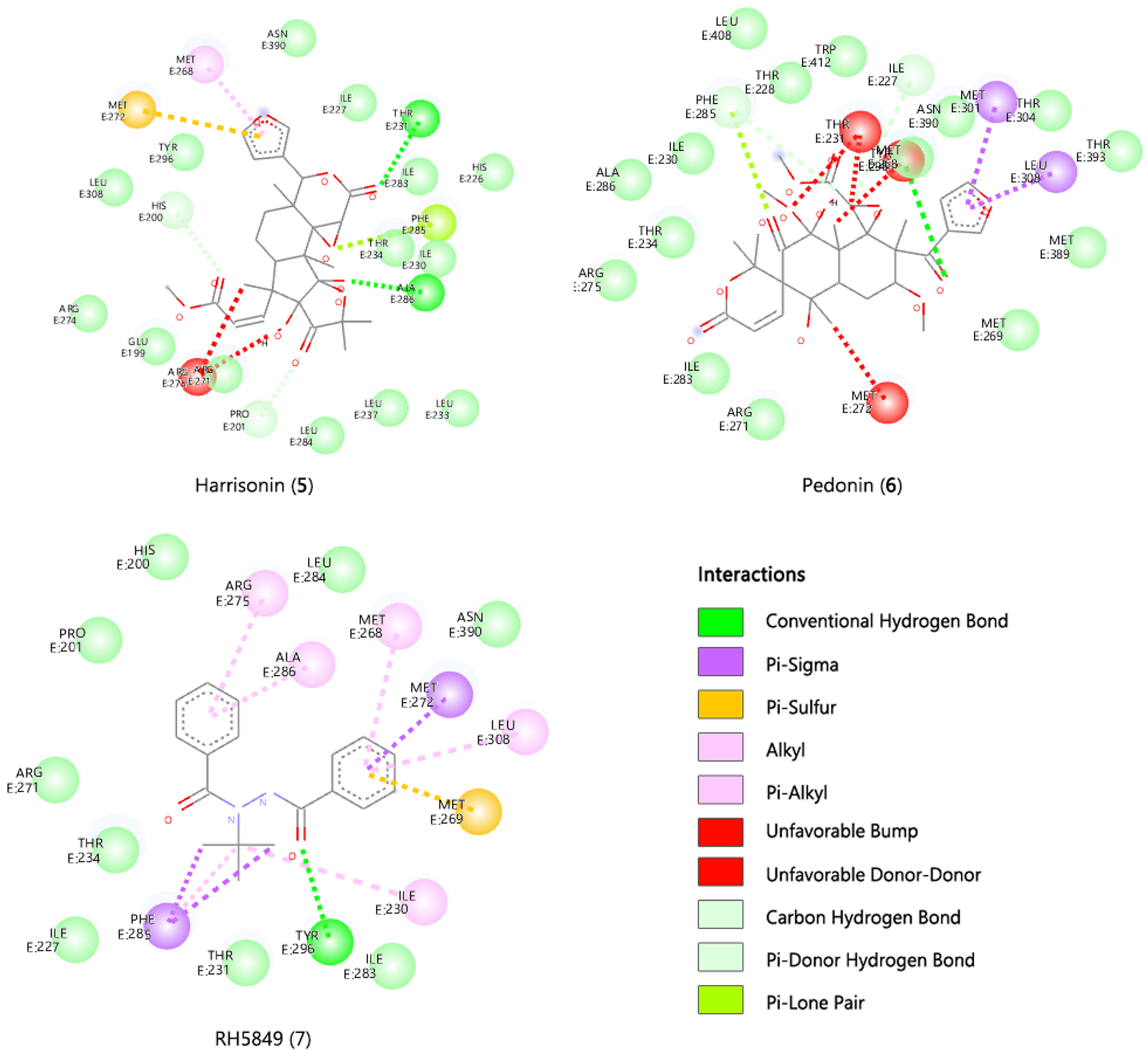
Figure 4. 2D diagrams showing interactions of amino acids in the receptor pocket docked with the most favourable pose of the commercial insecticide RH5849 at the EcR 1z5x receptor pocket.
Conclusion
In the study, six limonoids were selected and subjected to molecular docking studies. The compounds displayed interaction with EcR protein 1z5x LBP. The findings have illustrated the fact that limonoids have the potential to interfere with normal EcR protein function of Aedes aegypti L larvae by binding with the amino acid residues in the EcR active site. Tice rule of insecticide likeliness also indicated that compound 1 and 2 can be potential candidates as ecdysteroid agonists. The apparent correlation between the predicted binding energy, dissociation constants and the experimental larvicidal activities of the selected limonoids strongly suggests that these compounds or their variants could be applied in mosquito management programs against Aedes aegypti L. Also, since permanent activation of EcRs by certain compounds has been reported for inhibiting metamorphosis process, it is possible that the degraded limonoids (1 and 2), possessing a strong binding affinity could also serve as agonists against EcR protein and can be used for the development of potent, new, species specific insecticides. However, in-vitro studies should be conducted to understand the effect of promising inhibitors of molecular docking study, and the feasibility of using these inhibitors in the fields.
Declarations
Acknowledgment
The author acknowledges and appreciates the Pwani University Council for allowing him to spend his sabbatical leave as a visiting professor at the Technical University of Mombasa.
Ethics Statement
Not relevant
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Rodrigues NB, Godoy RSM, Orfano AS, Chaves BA, Campolina TB, Costa BDA. Brazilian Aedes aegypti as a Competent Vector for Multiple Complex Arboviral Coinfections. J. Infect. Dis. 2021; 224(1): 101-108.
- Iwamura T, Guzman-Holst A, Murray KA. Accelerating Invasion Potential of Disease Vector Aedes aegypti under Climate Change. Nat. Commun. 2020; 11: 21-30.
- Colon-González FJ, Sewe MO, Tompkins AM, Sjodin H, Casallas A, Rocklov J, Caminade C. Projecting the Risk of Mosquito-Borne Diseases in a Warmer and More Populated World: A Multi-Model, Multi-Scenario Inter-comparison Modelling Study. Lancet Planet. Health. 2021; 5: 404-414.
- Pereira Filho AA, Pessoa GCD, Yamaguchi LF, Stanton MA, Serravite AM, Pereira RHM, Neves WS, Kato MJ. Larvicidal Activity of Essential Oils from Piper Species Against Strains of Aedes aegypti (Diptera: Culicidae) Resistant to Pyrethroids. Front Plant Sci. 2021; 12(4): 685-864.
- Conway MJ, Haslitt DP, Swarts BM. Targeting Aedes aegypti Metabolism with Next-Generation Insecticides. Viruses 2023; 15: 469, 1-14.
- Innocent E and Magadula JJ. Variations of anti-mosquito larvicidal constituents in the Harrisonia abyssinica species of Tanzania. Int. J. Biol. Chem. Sci. 2010, 4(1): 218-225.
- Silvério MRS, Espindola LS, Lopes NP, Vieira PC. Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules. 2020; 25(15): 3484–3526.
- Pohlit AM, Rezende AR, Baldin ELL, Lopes NP, Neto VFA. Plant Extracts, Isolated Phytochemicals, and Plant-Derived Agents Which Are Lethal to Arthropod Vectors of Human Tropical Diseases – A Review. Planta Med. 2011; 77: 618–630.
- Dura´n-Pen MJ, Botubol-Ares JMC, Isidro GC, Hernandez GR. Degraded limonoids: biologically active limonoid fragments re-enhancing interest in Meliaceae and Rutaceae sources. Phytochem Rev. 2023; 22: 695–741.
- Ononamadu CJ, Abdalla M, Ihegboro GO, Jin L, Owolarafe TA, John TD, Qiang T. In silico identification and study of potential anti-mosquito juvenile hormone binding protein (MJHBP) compounds as candidates for dengue virus vector insecticides. Biochemistry and Biophysics Reports. 2021; 28: 101178, 1-12.
- Nguyen HH, Nguyen CT, Ngo HG, Nguyen GTT, Thuy PT, Setzer WN, Kuo P-C, Bui HM. Potential for Aedes aegypti Larval Control and Environmental Friendliness of the Compounds Containing 2‑Methyl-3,4-dihydroquinazolin-4-one Heterocycle ACS Omega 2023; 28(8): 25048-25058.
- Adline A, Selvaraj D. In silico molecular docking study of plant-based compounds from medicinal plant Lantana camara L. against Aedes aegypti L. protein International Journal of Mosquito Research 2022; 9(6): 97-106.
- Kim IIH, Pham V, Jablonka W, Goodman WG, Ribeiro JMC, Andersen JF. A mosquito hemolymph odorant-binding protein family member specifically binds juvenile hormone. J. Biol. Chem. 2017, 292(37): 15329 –15339.
- Devillers J, Lagneau C, Lattes A, Garrigues JC, Clémenté MM, Yébakima A. In silico models for predicting vector control chemicals targeting Aedes aegypti. SAR and QSAR in Environmental Research, 2014; 25(10): 805–835.
- Sandhya K, Sivakumar M, Vijayakumar AR. Larvicidal Activity and Molecular Docking Studies of Vitexicarpin from Vitex negundo Linn. J Young Pharm, 2021; 13(3): 223-228.
- Kiprop AK, Kiprono PC, Rajab MS and Wanjala, FME. Isolation and Characterization of Larvicidal Components against Mosquito Larvae (Aedes aegypti Linn) from Calodendrum capensi Thunb. Bull. Chem. Soc. Ethiop. 2005; 19(1): 145-148.
- Kiprop AK, Kiprono PC, Rajab MS, Kosgei MK. Limonoids as Larvicidal Components against Mosquito Larvae (Aedes aegypti Linn.). Z. Naturforsch., 2007; 62: 826-828.
- Carmichael JA, Lawrence MC, Graham LD, Pilling PA, Epa VC., Noyce L, Lovrecz G, Winkler DA, Pawlak-Skrzecz A, Eaton RE, Hannan GN, Hill RJ. The X-ray structure of a hemipteran ecdysone receptor ligand-binding domain: comparison with a lepidopteran ecdysone receptor ligand-binding domain and implications for insecticide design. J. Biol. Chem. 2005; 280: 22258-22269.
- de Oliveira DAB, da Silva AV, Niculau ES. Molecular Docking of Azadirachtin in Nuclear Ecdysone Receptor. Current Physical Chemistry, 2019; 9: 50-57.
- Pinto CPG. In silico Approaches for the Ecdysone Receptor Hemiptera: The First Step for Rational Pesticide Discovery. Int. J. Curr. Microbiol. App. Sci. 2019; 8(1): 261-270.
- Retnakaran A, Gelbic I, Sundaram M, Tomkins W, Ladd T, Primavera M, Feng Q, Arif B, Palli R, Krell P. Mode of action of the ecdysone agonist tebufenozide (RH-5992), and an exclusion mechanism to explain resistance to it. Pest Manag Sci. 2001; 57(10): 951-7.
- Yadav RP, Ibrahim KS, Gurusubramanian G, Kumar NS. In silico docking studies of non-azadirachtin limonoids against ecdysone receptor of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Med Chem Res. 2015, 24(6): 2621-2631
- Tice, CM. Selecting the right compounds for screening: Does Lipinski’s Rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manag Sci. 2001; 57: 3–16.
- Nakagawa Y, Henrich VC. Arthropod nuclear receptors and their role in molting. FEBS J. 2009; 276(21): 6128-6157.
- Billas IM, Moras D. Ligand-binding pocket of the ecdysone receptor. Vitamins & Hormones. 2005; 73: 101-129.
- Dhadialla TS, Carlson GR, Le, DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu. Rev. Entomol. 1998; 43: 545–546.
- Browning C, McEwen AG, Mori K, Yokoi T, Moras D, Nakagawa Y, Billas IML. Nonsteroidal ecdysone receptor agonists use a water channel for binding to the ecdysone receptor complex EcR/USP. J Pestic Sci. 2021; 46(1): 88-100.

 ETFLIN
Notification
ETFLIN
Notification







