Phytochemical Profiling, Cytotoxicity, and Antiproliferative Potential of Solenostemon monostachyus (Fabaceae) Leaves
by Emmanuel Eimiomodebheki Odion ★ , Daniel Akpe-Efiak Ambe, Kidochukwu Naomi Ifejika, Eravweroso Congrat Odiete, Chinyelu Clementina Osigwe
Academic editor: Pilli Govindaiah
Sciences of Phytochemistry 3(2): 72-81 (2024); https://doi.org/10.58920/sciphy0302244
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
11 May 2024
09 Jul 2024
11 Jul 2024
21 Jul 2024
Abstract: Solenostemon monostachyus (S. monostachyus) is a widely distributed and important herb in central and west Africa, traditionally used in treating various ailments, including tumors. This study aims to identify the phytoconstituents in the methanol leaf extract of S. monostachyus and evaluate the cytotoxic and anti-proliferative potentials of the methanol extract and its fractions (n-hexane, dichloromethane, and ethyl acetate). Preliminary phytochemical screening was conducted to determine different classes of phytochemical constituents in the powdered leaf. Phytoconstituents were identified from the methanol extract by chromatographic analysis (HPLC and GC-MS). The extract and fractions of S. monostachyus were tested against Raniceps raninus tadpoles and Sorghum bicolor radicles to evaluate their cytotoxic and growth suppression potentials. HPLC analysis revealed catechin, cyanogenic glycosides, flavanone, sparteine, sapogenin, and phytate. GC-MS analysis displayed (Z)-2,3-dihydroxypropyl 9-octadecenoic acid ester, 2-dodecyl-1,3-propanediol, 1-nitro-bicyclo[6.1.0]nonan-2-one, and furazano[3,4-b]pyrazine-5,6-diamine, N, N’-di(propynyl) as the prominent compounds. A cytotoxic effect was observed at 160 µg/mL, with a recorded 56.67 ± 3.33% mortality within 0.5 h, increasing to 100.00 ± 0.00% mortality of the tadpoles within 1 h of treatment. A concentration of 16 mg/mL of S. monostachyus extract significantly (p<0.05) exerted 56.15% (0.82 ± 0.08) suppression of the emerging radicles in 24 h, which later increased to 94.55% (1.10 ± 0.07) after 96 h. This indicates that S. monostachyus leaf extract contains phytochemicals with cytotoxic and growth-suppression potentials.
Keywords: Raniceps raninusSorghum bicolorCyanogenic glycosidesSparteine
Introduction
Cancer is a leading public health issue and one of the main causes of death globally, despite advancements in medical research and technology. In developed countries, the number of reported cases outweighs the recorded deaths, while in underdeveloped countries, many of the cases are not reported. It is estimated that cancer will contribute to 9.6 million deaths by 2024 (1). This alarming figure highlights the urgent need for preventive measures or medicines that could be used for the management or treatment of this condition. Plants are a reservoir of yet-to-be-identified compounds with varying pharmacological potentials (2).
S. monostachyus, from the family Lamiaceae, is a well-distributed and crucial herb in Sub-Saharan Africa, especially in Nigeria, Ghana, Sierra Leone, Cameroon, and Gabon. It is also known as monkey’s potato and grows annually as a green weed to a height of 100 cm. S. monostachyus is an annual succulent, aromatic, erect, and branched plant. The flowers are violet and have lengthy inflorescences (3). The leaves are oppositely arranged, simple, stipulate absent, with a petiole 1.5 to 4.0 cm long. The blade is ovate, 5.0 to 9.0 cm x 3.0 to 6.0 cm, cuneate at the base, obtuse to acute at the apex, with a crenate margin, puberulous and gland-dotted underneath, with separated veins (4). Synonyms of S. monostachyus include Coleus africanus Benth., Ocymum monostachyum P. Beauv., Plectranthus palisoti Benth., Solenostemon lateriticola A. Chev., and Solenostemon ocymoides Schum. & Thonn (5).
Some phytochemicals reported in the leaves include alkaloids, flavonoids, saponins, tannins, and glycosides (6, 7). HPLC analysis identified apigenin, hesperidin, myricetin, morin, caffeic acid, ferulic acid, and quercetin as the major constituents in the leaves extract (8, 9). Analysis by GC-MS of the constituent oils in the aerial parts of S. monostachyus identified limonene, neral, trans-verbenol, and decanal as prominent bioactive compounds (9). A decoction from the leaves is traditionally used to manage tumors, arthritis pain, and rheumatism. The leaves have also been reported to have body temperature-lowering effects, sedative, antiemetic, and laxative potentials. Eye, pulmonary, and parasitic skin infections are treated with the leaves extract. It is also used as an antidote against venomous stings and bites (10). Pharmacologically, the leaves are known to have analgesic and anti-inflammatory actions (11), anti-ulcer (12), antioxidant (13), antimicrobial (14), antinociceptive, antiplasmodial, antipyretic (4), antidiabetic, hypolipidemic (3), and antihypertensive effects (15). A literature search has shown a paucity of information on the cytotoxic and anti-proliferative actions of the methanol extract and fractions of the leaves of S. monostachyus. Identifying the phytochemicals with cytotoxic and anti-proliferative potentials could lead to the discovery of new compounds. Thus, this study intends to report the phytochemicals in the leaves of S. monostachyus in relation to the cytotoxic and anti-proliferative potentials in the methanol extract and its fractions.
Materials and Methods
Collection, Identification, Extraction, and Fractionation
The leaves of S. monostachyus were collected in October 2023 at the University of Benin, Ugbowo Campus, located at latitude 6° 20' 1.32"N and longitude 5° 36' 0.53"E. The plant was identified by Prof. H.A. Akinnibosun of the Department of Plant Biology and Biotechnology, University of Benin. Herbarium number UBH-5392 was issued, and the specimen was kept in the Herbarium.
S. monostachyus leaves were carefully separated from the stem, air-dried for fourteen days, pulverized using an electrical milling machine, and placed in an airtight container until used. Two hundred grams of the pulverized leaves was macerated with 99.99% methanol (2000 mL) for three days. The solvent was subsequently decanted and passed through size 1 filter paper. Filtrate obtained was concentrated at a temperature of 60°C in vacuum and forty grams (40 g) of the extract was obtained.
Extract (30 g) was solubilized in methanol (30 mL) and water (120 mL) respectively, and fractionated with 4 x 100 mL of n-hexane. The n-hexane fractions collected were pulled together and concentrated in a vacuum at 50°C. This were repeated for dichloromethane and ethylacetate fractions, which were stored in the refrigerator at 4°C until used.
Phytochemical Screening
The phytochemicals in the powdered leaves were evaluated using the method described by Sofowora (16) and Trease and Evans (17). Phytochemicals determined include alkaloids, flavonoids, tannins, glycosides (cardiac and cyanogenic) triterpenoids and saponins.
High Pressure Liquid Chromatography (HPLC) Analysis
The method used for this analysis involved the use of ShimadzuLC-10AD dual binary pumps, Shimadzu CTO-10AS column oven and UV-Vis detector (Shimadzu Prominence SPD-20A) HPLC separation of S. monostachyus extract was achieved with a C-12 normal phase column (200 mm length x 4.8 µm internal diameter x 5 µm Phenomenex, Gemini). Mobile phase (A and B) with a flow rate of 0.8 mL/min, consist of acetic acid-acidified de-ionized water (pH 2.8)-A and acetonitrile-B. Prior to each analysis, re-equilibration was achieved using solvent B (5%) for 20 min after each injection with 38°C as the set column temperature and injection volume of 20 µL. Wavelength for detection was set at 280 nm, identification and quantification were performed by comparing retention times and peak areas with reference standard using the method of external standards to construct calibration curve for the analysis. Elution was by gradient: 0-5 min, 5-9% solvent B; 5-15 min, 9% solvent B; 15-22 min, 9-11% solvent B; 22-38 min, 11-18% solvent B; 38-43 min, 18-23% solvent B; 43-44 min, 23-80% solvent B; 44-45 min, 80-90%, solvent B; 45-55 min, 100% solvent B (18).
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Analysis of the methanol leaves extract of S. monostachyus was performed using an Agilent USA gas chromatograph (7890A) coupled to a mass spectrometer (5975C inert MSD) with a triple-axis detector, equipped with a 10 mL syringe auto-injector. Phenyl methyl siloxane (5%) and helium gas were used as the stationary and mobile phases, respectively, while a capillary column (Agilent 19091-433HP-5Ms) with the following specifications: length 30 mm, internal diameter 0.2 µm, thickness 250 µm, and pressure of 16.2 psia, was used. A sample size of 1 µL was injected in split mode with a split ratio of 1:50. The GC operating conditions were as follows: the inlet temperature was kept at 280°C, while the oven temperature was maintained at 50°C for 2 min before it was ramped at 20°C/min to 100°C. The rate of temperature increase was maintained while the temperature was increased from 100°C to 250°C and held for 5 min. The mass spectrometer used an electron impact mode of 70 eV ionization energy and was scanned over 45 to 700 Daltons. MS solution software from the manufacturer was used to acquire data, and compounds were identified by comparing their mass spectra with existing data from the National Institute of Standards and Technology library (NIST 11) (18).
Cytotoxicity Test
The cytotoxic effects of the methanol extract and fractions of S. monostachyus leaves were measured using Raniceps raninus (tadpole-fish). A 50 mL pipette (internal diameter 20 mm) was used to select ten tadpoles, which were then placed into a 50 mL beaker with 15 mL of water collected from their natural habitat. Each 50 mL beaker containing 15 mL of water from the natural habitat was filled to a capacity of 49 mL with 34 mL of distilled water. Using 1 mL of 1, 2, 4, 8, and 16 mg/mL of methanol leaves extract of S. monostachyus, the final volume was adjusted to 50 mL, resulting in 20, 40, 80, 160, and 320 μg/mL concentrations, respectively. The negative control consisted of distilled water. Three duplicates of the experiment were run for each group. The experiments were repeated for the n-hexane, dichloromethane, and ethyl acetate fractions. Tadpole mortality was monitored for 24 h and expressed as the mean ± standard error of the mean (19).
Growth-Suppression Activity
The suppression of growth by the methanol extract and fractions of S. monostachyus leaves was determined by measuring the growth inhibition of Sorghum bicolor radicles. Cotton wool and size 1 Whatman filter paper were placed inside glass Petri dishes. Ten milliliters of 1, 2, 4, 8, and 16 mg/mL of extract were applied to each Petri dish, along with distilled water, serving as a negative control. Twenty viable Sorghum bicolor seeds were spread out on each plate and kept in the dark. The length of the emerging radicles was measured at 24, 48, 72, and 96 h. The experiment was repeated for the fractions and conducted in triplicate (19).
Statistical Analysis
Analysis of variance (ANOVA) was utilized in data analysis, level of significance set at p<0.05 and multiple group comparison was performed using Dunnett Post Hoc test. Data were plotted and analyzed using Graphpad version 6.0.
Results
Yields and Phytochemicals
Extracting the powdered leaves of S. monostachyus with 99.99% of methanol yielded 14.50%. Fractionation of the crude extract with n-hexane, dichlorometane and ethylacetate yielded 20.00%, 15.40% and 13.60% of the fractions respectively.
Table 1. Percentage yield of extract and fractions from S. monostachyus leaves.
S/N | Extract/Fractions | Percentage yield (%) |
1. | Methanol | 14.50 |
2. | N-hexane | 20.00 |
3. | Dichloromethane | 13.40 |
4. | Ethylacetate | 15.60 |
Screening of the powdered leaves of S. monostachyus for phytochemicals revealed alkaloid, flavonoid, glycosides, saponin, steroid and tannin (see Table 2).
Table 2. Phytochemical screening of the powdered leaves of S. monostachyus.
Phytochemicals | Inference |
Alkaloid | + |
Cyanogenic glycoside | + |
Cardiac glycoside | + |
Flavonoid | + |
Tannin | + |
Triterpenoids | - |
Saponin | + |
Steroid | + |
Note: (+) = positive and (-) = negative.
Eighteen compounds were identified and quantified from the methanol leaves extract of S. monostachyus (Table 3). Six of these compounds contains 59.57% of the total number of compounds determined. They include catechin (33.3344 µg/mL), flavonone (18.9364 µg/mL), sparteine (18.2949 µg/mL), cyanogenic glycoside (13.4836 µg/mL), sapogenin (13.1162 µg/mL) and phytate (11.9786 µg/mL). These phyto-compounds were obtained from standards that matched compounds in the methanol leaves extract of S. monostachyus. Also compounds determined by HPLC analysis are non-volatile in nature.
Forty-seven compounds were identified (Table 4) from the GC-MS analysis of the methanol leaves extract of S. monostachyus. Nine of these compounds are prominent (within percentage area of 3 to 15%), they include. (Z)-2,3-dihydroxypropyl-9-octadecenoic acid ester (6.03%); cis-11-Eicosenoic acid (3.56%); 1,3-Propanediol, 2-dodecyl (4.62%); cis-9-Hexadecenal (3.07%); 3,8-Dioxatricyclo[5.1.0.0(2, 4)]octane, 4-ethenyl- (3.23%); 1-Nitro-bicyclo[6.1.0]nonan-2-one (7.07%); N,N'-di(propynyl)furazano[3,4-b]pyrazine-5,6-diamine (4.79%); N-(3-chlorophenyl)-3-[4-(2-hydroxyethyl)-piperazin-1-yl]-Propionamide (3.17%); and 3-Oxabicyclo[3.3.0]octane-2,7-dione, 4-methoxy-,trans- (3.35%). Compounds identified by GC-MS technique are volatile in nature (18).
Table 3. HPLC profiling of methanol leaves extract of S. monostachyus.
S/N | Components | Retention Time | Peak Area | Concentration (µg/ml) |
1 | Kaempferol | 0.156 | 2917.6373 | 3.3896 |
2 | Steroid | 2.400 | 12985.2850 | 8.9905 |
3 | Ephedrine | 4.103 | 5482.6536 | 6.8065 |
4 | Catechin | 6.043 | 26859.1547 | 33.3344 |
5 | Cyanogenic glycosides | 10.366 | 19241.1074 | 13.4836 |
6 | Narigenin | 12.966 | 5755.4276 | 7.3977 |
7 | Dihydrocystine | 15.460 | 4429.6532 | 5.6985 |
8 | Quercetin | 17.966 | 10769.1219 | 0.7027 |
9 | Aphyllidine | 20.313 | 12189.4039 | 8.2696 |
10 | Ammodendrine | 22.726 | 8854.6948 | 4.3379 |
11 | Tannin | 25.660 | 9351.2137 | 5.0901 |
12 | Flavonones | 27.530 | 11039.9056 | 18.9364 |
13 | Cardiac glycoside | 29.860 | 4720.5040 | 5.8603 |
14 | Sparteine | 33.010 | 14741.1314 | 18.2949 |
15 | Flavone | 34.480 | 4528.4416 | 5.6219 |
16 | Ribalinidine | 36.880 | 6449.2172 | 8.0040 |
17 | Phytate | 39.200 | 9651.7528 | 11.9786 |
18 | Sapogenin | 44.166 | 10565.1153 | 13.1162 |
Table 4. Chemical composition of methanol leaves extract of S. monostachyus analyzed by GC-MS. Note: RT = retention time, PC = percentage composition, MW = molecular weight, and MF = molecular formula.
S/N | Compounds | RT | PC | MF | MW |
1 | Oleic Acid | 2.285 | 2.25 | C18H34O2 | 282.468 |
2 | Palmitoleic acid | 2.369 | 3.56 | C16H30O2 | 254.414 |
3 | 9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester | 2.566 | 6.03 | C21H40O4 | 356.5399 |
4 | Ascorbyl Palmitate | 2.876 | 1.67 | C22H38O7 | 414.539 |
5 | cis-10-Nonadecenoic acid | 2.961 | 2.53 | C19H36O2 | 296.4879 |
6 | cis-11-Eicosenoic acid | 3.130 | 3.56 | C20H38O2 | 310.51 |
7 | cis-Vaccenic acid | 3.355 | 3.46 | C18H34O2 | 282.461 |
8 | 1,3-Propanediol, 2-dodecyl | 3.552 | 4.62 | C15H32O2 | 244.413 |
9 | cis-9-Hexadecenal | 3.862 | 3.07 | C16H30O | 238.41 |
10 | 15-Hydroxypentadecanoic acid | 4.116 | 2.82 | C15H30O3 | 258.3969 |
11 | Z-8-Octadecen-1-ol acetate | 5.778 | 1.50 | C20H38O2 | 310.514 |
12 | Tetradecane | 6.144 | 0.16 | C14H30 | 198.314 |
13 | Benzamide, N-(4-cyanomethylphenyl)-2-nitro- | 6.398 | 0.52 | C15H11N3O3 | 281.27 |
14 | 5,9-Dimethyl-9-decen-3-ol | 6.707 | 0.19 | C12H24O | 184.32 |
15 | Dodecanoic acid, methyl ester | 6.989 | 1.24 | C14H28O2 | 214.3443 |
16 | p-Butyryloxybenzaldehyde | 7.327 | 0.28 | C11H12O3 | 192.2112 |
17 | 15-Hydroxypentadecanoic acid | 7.440 | 0.73 | C15H30O3 | 258.40 |
18 | Diethyl Phthalate | 7.750 | 0.27 | C12H14O4 | 222.24 |
19 | Acetic acid, 2(1,3-dihydro-5-nitro -1,3-dioxo-isoindol-2-yl)phenyl ester | 8.172 | 0.09 | C16H11N2O4 | 295.27 |
20 | Methyl tetradecanoate | 8.398 | 1.40 | C15H30O2 | 242.3975 |
21 | N-Methoxy-1-ribofuranosyl-4-imidazolecarboxylic amide | 8.567 | 0.92 | C10H15N3O6 | 273.24 |
22 | 6H-Purin-6-one, 3,7-dihydro-3-methyl- | 9.355 | 0.37 | C6H6N4O | 150.14 |
23 | Muscimol | 9.891 | 0.15 | C4H6N2O2 | 114.104 |
24 | 1,4-Dithiin, 2,3-dihydro-5,6-dimethyl-, 1,1,4,4-tetraoxide | 10.116 | 0.07 | C6H10O4S2 | 210.271 |
25 | Pentadecanoic acid, 14-methyl-, methyl ester | 10.482 | 2.95 | C17H34O2 | 270.5 |
26 | (Z)-9-Hexadecenoic acid, methyl ester, | 10.820 | 0.41 | C17H32O2 | 268.4348 |
27 | 2-O-Mesyl arabinose | 12.032 | 1.97 | C6H12O7S | 228.22 |
28 | Mitozolomide | 12.229 | 0.85 | C7H7ClN6O2 | 242.62 |
29 | 12.370 | 3.23 | C8H10O2 | 138.16 | |
30 | 2,2-diphenyl-2H-1-Benzopyran, | 12.708 | 1.68 | C21H18O | 286.4 |
31 | 2-Furanilide | 12.989 | 1.75 | C11H9NO2 | 187.19 |
32 | L-Arginine, methyl ester | 13.102 | 0.98 | C7H16N4O2 | 188.32 |
33 | 1-Nitro-bicyclo[6.1.0]nonan-2-one | 13.806 | 7.07 | C9H13NO3 | 183.20 |
34 | N,N'-di(propynyl)furazano[3,4-b]pyrazine-5,6-diamine, | 14.201 | 4.79 | C10H8N6O | 228.21 |
35 | 6-tert-butyl-4-methyl-2-phenylpyrazolo[3,4-b]pyridin-3(2H)-one | 14.482 | 1.57 | C18H20N2O | 280.38 |
36 | Dimethylmalonic acid, 4-acetylphenyl undecyl ester | 14.567 | 1.69 | C24H36O5 | 404.5 |
37 | N-[3-Cyano-4-methyl-5-(piperidine-1-carbonyl)-thiophen-2-yl]-2,3,3,3-tetrafluoro-2-methoxy-propionamid | 14.736 | 0.89 | C16H17F4N3O3S | 407.4 |
38 | Benzoic acid, 2,5-bis(trimethylsiloxy)-, trimethylsilyl ester | 14.933 | 2.47 | C15H24ClN2O2 | 299.84 |
39 | N-(3-chlorophenyl)-3-[4-(2-hydroxyethyl)-piperazin-1-yl]-Propionamide | 15.102 | 3.17 | C16H30O4Si3 | 370.66 |
40 | 3-Oxabicyclo[3.3.0]octane-2,7-dione, 4-methoxy-,trans- | 15.412 | 3.35 | C8H12O4 | 172.18 |
41 | 4-Heptanone,dimethylhydrazone | 15.918 | 1.54 | C9H20N2 | 156.27 |
42 | Hexahydro-1,2,4-triazino[5,6-E][1,2,4]-triazine-3,6-dione | 16.116 | 2.26 | C4H8N6O2 | 172.15 |
43 | Methyl 6-O-[1-methylpropyl]-.beta.-d-galactopyranoside | 16.67 | 0.95 | C11H17O6 | 245.28 |
44 | 1-(ethenyloxy)octadecane | 16.820 | 0.26 | C20H40O | 296.5310 |
45 | 2-(4-methylphenyl)indolizine | 17.384 | 0.45 | C15H13N | 207.27 |
46 | Perhydro-htx-2-one, 2-depentyl-, acetate ester | 18.567 | -0.66 | C16H27NO3 | 281.39 |
47 | Nonanoic acid | 18.736 | 0.79 | C9H18O2 | 158.24 |
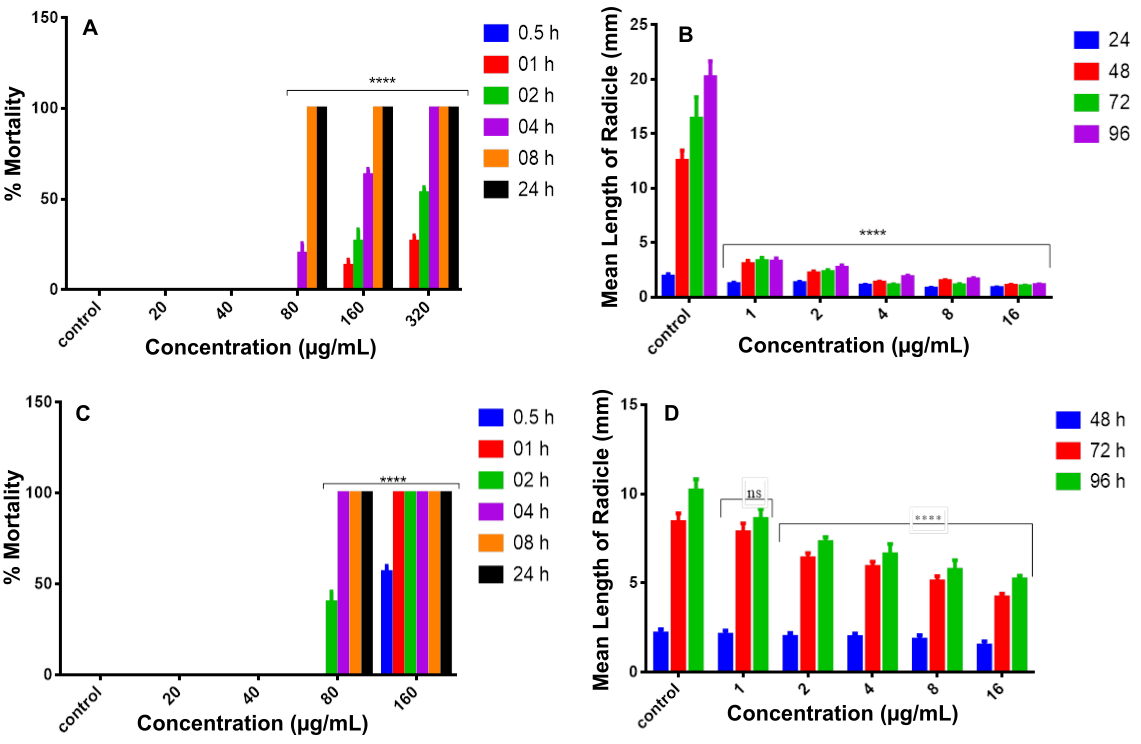
Figure 1. Cytotoxicity and antiproliferative effect of methanol leaves extract on (A) R. raninus and (B) S. bicolor radicles, and dichloromethane fraction on (C) R. raninus and (D) S. bicolor radicles. Each bar represents mean ± SEM. Data with ****are significant at p<0.0001 where ns = not significant and n=10.
Cytotoxicity and Antiproliferative Effect of Methanol Extract
The methanol leaves extract of S. monostachyus was screened for cytotoxic activity using the Raniceps raninus in vitro model. The extract showed potent cytotoxic activity against the R. raninus tadpoles. At a concentration of 80 μg/mL, the extract caused a mortality of 20.00 ± 5.8% within 4 h and 100.00 ± 0.00% within 8 h. The most cytotoxic effects were observed at 320 µg/mL, with 53.3 ± 3.33% mortality after 2 h and 100.00 ± 0.00% death after 4 h. No mortality was observed in the controls (Figure 1A).
Methanol leaves extract of S. monostachyus was observed to elicit concentration-dependent suppression in the length of the emerging radicles. The 1 mg/mL concentration significantly (p<0.05) caused 36% (1.18 ± 0.16) suppression of the radicles at 24 h, which increased to 83.91% (3.25 ± 0.3) in 96 h. The 16 mg/mL concentration significantly (p<0.05) exerted 56.15% (0.82 ± 0.08) suppression of the emerging radicles in 24 h that increased to 94.55% (1.10 ± 0.07) after 96 h (see Figure 1B).
Cytotoxicity and Antiproliferative Effect of Dichloromethane Fraction
The dichloromethane fraction of leaves extract of S. monostachyus was screened for cytotoxic activity using the Raniceps raninus in vitro model. At a concentration of 80 μg/mL, the extract caused a mortality of 40.00 ± 5.77% within 2 h and 100.00 ± 0.00% within 4 h. The most cytotoxic effects were observed at 160 µg/mL, with 56.67 ± 3.33% mortality within 0.5 h and 100.00 ± 0.00% death within 1 h. No mortality was observed in the controls (Figure 1C).
The dichloromethane fraction of the leaves extract of S. monostachyus was observed to elicit concentration-dependent suppression in the length of the emerging radicles. The 1 mg/mL concentration non-significantly (p<0.05) caused 3.67% (2.10 ± 0.24) suppression of the radicles in 48 h, which increased to 15.69% (8.60 ± 0.56 ) in 96 h. The 16 mg/mL concentration significantly (p<0.05) exerted 31.19% (1.50 ± 0.23) suppression of the emerging radicles in 48 h that increased to 48.92% (5.21 ± 0.21) after 96 h (Figure 1D).
Discussion
The extraction method used in this study was simple and convenient. It involved inducing mass transfer with shaking until the powdered leaves and solvent (methanol) reached equilibrium. Though it is a well-known technique, it may require a second step for the concentration of the extract (20). The extract obtained from this process contains polar, semi-polar, and non-polar compounds. Fractionation aids in the partial purification process of the crude extract, leading to the separation of the constituents into polar, semi-polar, and non-polar compounds. In this study, the n-hexane fraction showed the highest yield (20.00%), implying that non-polar compounds are more abundant in the leaves of S. monostachyus, while the dichloromethane fraction showed the lowest yield (13.40%), indicating the presence of semi-polar constituents.
Screening of the powdered leaves of S. monostachyus for phytochemicals revealed alkaloid, flavonoid, glycosides, saponin, steroid and tannin (Table 2). Theses are in agreement with the work done by Enin and co-workers, who reported alkaloids, glycosides, tannins and saponins in ethanol leaves extract of S. monostachyus (21). These phytochemicals are made-up of varying atoms, combined to form moieties that were originally made for the defence of the plant from predators but have found use in medicine.
There is recurring evidence supporting the global search for medicinal compounds in plants, as they form the basis of many reported pharmacological activities (22). This screening showed that the powdered leaves of S. monostachyus are rich in these phytoconstituents, and their presence may be implicated in many of its reported traditional uses. Flavonoids have several benefits relevant to this study, including antioxidant, anti-inflammatory, and anticancer effects (23). Steroids in medicinal plants have been linked to antitumor (24) and immune response-modulating agents (25), and alkaloids have been recognized as cancer-preventive agents (26).
Eighteen compounds were identified and quantified from the methanol leaves extract of S. monostachyus (Table 3). Six of these compounds contains 59.57% of the total number of compounds determined. They include catechin (33.3344 µg/ml), flavonone (18.9364 µg/ml), sparteine (18.2949 µg/ml), cyanogenic glycoside (13.4836 µg/ml), sapogenin (13.1162 µg/ml) and phytate (11.9786 µg/ml). These phyto-compounds were obtained from standards that matched compounds in the methanol leaves extract of S. monostachyus. Also, compounds determined by HPLC analysis are non-volatile in nature.
Catechin is a natural flavonoid known for its antioxidant, anti-inflammatory, and anti-carcinogenic activities. It mechanism of action include the inhibition of proliferation and growth of cancerous cells, scavenging free radical, suppressing metastasis, improving immunity and regulating signal pathways (27). Sparteine is a naturally occurring alkaloid exert more intense anti-proliferative activities on DoTc2 cells, though it shows marginal cytotoxicity on cervical cells, making it a potential candidate for chemotherapy in cervical cancer treatment (28). Kaempferol is a natural polyphenol with anticancer and antioxidant properties (29), exert it effect by modulating cell signal pathways, induction of apoptosis and cause cell cycle arrest in cancerous cell (30).
Forty-seven compounds were identified (Table 4) from the GC-MS analysis of the methanol leaves extract of S. monostachyus. Nine of these compounds are prominent (within percentage area of 3 to 15%), they include. (Z)-2,3-dihydroxypropyl-9-octadecenoic acid ester (6.03%); cis-11-Eicosenoic acid (3.56%); 1,3-Propanediol, 2-dodecyl (4.62%); cis-9-Hexadecenal (3.07%); 3,8-Dioxatricyclo[5.1.0.0(2, 4)]octane, 4-ethenyl- (3.23%); 1-Nitro-bicyclo[6.1.0]nonan-2-one (7.07%); N,N'-di(propynyl)furazano[3,4-b]pyrazine-5,6-diamine (4.79%); N-(3-chlorophenyl)-3-[4-(2-hydroxyethyl)-piperazin-1-yl]-Propionamide (3.17%) and 3-Oxabicyclo[3.3.0]octane-2,7-dione, 4-methoxy-,trans- (3.35%). Compounds identified by GC-MS technique are volatile in nature.
The detection of poly-unsaturated fat in the methanol leaves extract of S. monostachyus showed the likelihood of the extract to selectively reduce viability in MCF-7 cells and A549 cells (31). Oleic acid has been reported to significantly inhibit the proliferation of cells and tumor growth in endometrial cancer cells, it thought to exert its effect through the PTEN/AKT.mTOR pathway (32). Ascorbyl palmitate, an ester formed from ascorbic acid and palmitic acid, creating a fat-soluble ascorbic acid used to preserve food. It reduces the growth of Human Umbilical Vein Endothelial Cells by acting on the early or late stages of apoptosis through up-regulation of caspase 3,9 and down-regulation of the Bcl-2 ratio. Also, chromatin fragmentation was observed from the DAPI stains' morphology (33). Vaccenic acid inhibits proliferation and induces apoptosis in nasopharyngeal carcinoma cells (34).
Several medicinal plant species possess cytotoxic and antitumor properties against different types of cancer cells (35). These findings highlight the potential of the S. monostachyus leaves extract in treating tumors and cancerous tumors. The presence of the secondary metabolites identified by the GC-MS and HPLC in the methanol extract is responsible for the observed activity. The cytotoxic and anti-proliferative effects were seen to be dose-dependent. Higher concentrations resulted in a shorter duration of onset of the cytotoxic effect. The crude extract and dichloromethane fraction exhibited more cytotoxic effects than the crude methanol extract. This indicates that the cytotoxic constituents in the dichloromethane fraction are lipophilic. The effect of the extract was seen to be lethal to the emerging radicles at higher concentrations. The partitioning of the crude extract with dichloromethane resulted in the reduction of anti-proliferative effect of the dichloromethane fraction. The results conform with previous studies that reported the cytotoxic effect of compounds identified in the plant, including Kaemferol (36), quercetin and sparteine (37). The presence of catechin, flavone, flavonone, kaempferol, narigenin and quercetin in the methanol leaves extract indicate that these compounds with reported antioxidant potential, could exert its cytotoxic and anti-proliferative effects by either donating electron or hydrogen atom to quench rampaging free radical thus apoptosis or suppression of cell growth occurs.
Conclusion
This study provides information on the phytochemical constituents of S. monostachyus leaves, particularly the presence of alkaloids, flavonoids, glycosides, saponins, steroids, and tannins. The HPLC and GC-MS analyses have further provided details on the identity of these compounds. These phytochemicals have been associated with various pharmacological activities such as antioxidant, anti-inflammatory, antiviral, neuroprotective and anticancer effects, including the observed cytotoxic and anti-proliferative effects. These findings suggest the potential pharmacological activities of this plant and could be a useful source of these important compounds identified in future.
Declarations
Acknowledgment
Special thanks to the Head of Department of Chemical Engineering, University of Ilorin, for providing assess for the GC-MS analysis and Dr David Ogochukwu of Docchy Analytical Laboratories and Environmental Services Limited for running the HPLC analysis.
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Siegel, R.L, Miller, K.D, Wagle, N.S., and Jemal, A. Cancer statistics, (2023). CA Cancer Journal for Clinicians. 73 (1):17-48. doi: 10.3322/caac.21763.
- Khoo, H.E, Azlan, A., Kong, K,W and Isail A. (2016). Phytochemicals and Medicinal Properties of Indigenous Tropical Fruits with Potential for Commercial Development. Evidence-Based Complementary and Alternative Medicine. 1-20 http://dx.doi.org/10.1155/2016/7591951
- Okokon, J. E, Amazu, L. U., and Nwidu, L. L. (2015). Anti-diabetic and hypolipidaemic activities of Solenostemon monostachyus. Journal of Herbal Drug. 6(2):83-90.
- Umoh, R.A., Johnny, I.I., Udoh, A.E., Andy, N.A., Essien, A.C., Udoh, I.J., Emeh, W.E. and Umanah, O.E. (2021). Micromorphological and Pharmacognostic Studies of Leaf and Stem of Solenostemon monostachyus P.Beauv (Lamiaceae). Journal of Complementary and Alternative Medical Research.16(4): 230-240.
- Dokosi, O.B. (1998): Herbs of Ghana. Ghana University Press, Accra, Ghana.
- Obichi, E.A, Monago, C.C. and Belonwu, D.C (2015). Nutritional Qualities and Phytochemical Compositions of Solenostemon monostachyus (Family Lamiaceae). Journal of Environment and Earth Science. 5, 3, 105-111.
- Afolabi, I. S., Jolaoluwa, A. F., Awogbindin, V. O., Amosun, P. T. (2016). Phytonutrients and bioactive compounds in the leaves of Solenostemon monostachyus. Planta Medica; 82(S 01): S1-S381. doi: 10.1055/s-0036-1596424
- Afolabi, I.S., Uchendu J.O., and Mustapha, O.S. (2021) Antioxidant And Phytochemical Qualities of Solenostemon monostachyus (SoleMon). Rasayan Journal of. Chemistry., 14(3), 2154-2160.
- Okhale, S.E., Imoisi, C., Ode, S. S., James, J.G., and Lateef, A. (2023) Chromatographic analysis and antioxidant activities of the aerial part essential oil of Solenostemon monostachyus (P. beauv). International Journal of Pharmacognosy. 10(2): 236-43. doi: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.10(2).236-43.
- Burkill, H.M. (1985). The useful plants of west tropical Africa, Vol 3.
- Okokon, J.F., Davis, K., and Nwidu L.L. (2016). Anti-inflammatory and antinociceptive activities of Solenostemon monostachyus aerial part extract in mice. Avicenna Journal of Phytomedicine. 6(3): 284–294.
- Amazu, L.U., Antia, B.S., and Okokon, J.E. (2015). Antiulcer activity of S monostachyus. The Journal of Phytopharmacology. 4:97–101.
- Okoko, T., and Ere, D. (2012). Antioxidant activities of Solenostemon monostachyus leaf extract using in vitro methods. Scientific Research and Essays. 7:621–626.
- Ekundayo, E. O., and Ezeogu, L.I., (2006). Evaluation of antimicrobial activities of extracts of five plants used in traditional medicine in Nigeria. International Journal of Tropical Medicine.1:93–96.
- Fidele, K. Z., Andre, K. B., Yao, D. J., and Michel, O.A. (2012). Action of hydroethanolic leaves extract of Solenostemon monostachyus (Lamiaceae) on cardiovascular system of mammalians: blood pressure lowering effects. International Journal of Pharmacy and Biological Science. 2:310–320.
- Sofowora, A. (1993) Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa Edition. Spectrum Books Ltd., Nigeria, 150-156.
- Evans WC. (2009). Trease and Evans Pharmacognosy. 16th Edition. Elsiver Ltd. 135-147.
- Odion EE, Nwigwe GN, Ambe DA, Nnamani MN, Osigwe CC, Odiete EC, Iyanyi LU. (2024). Phytochemical Profiling of Passiflora edulis Vines. Science of Phytochemistry. 3, 1, 11-19. https://doi.org/10.58920/sciphy0301219]
- Ambe, D. A., and Ayinde, B. A. (2023). Lonchocarpus griffonianus (Baill) Dunn (Fabaceae) Attenuates Growth Proliferation: An index of usage in cancer management. Nigerian Journal of Applied Science. 41:16–22.
- Durovic, S., Dominguez, R., Pateiro, M., Teslic N., Lorenzo, J.M., Pavlic B. (2022). Chapter 9 - Industrial hemp nutraceutical processing and technology, Edited: Milica Pojic, M., Tiwari, B.K., Industrial Hemp, Academic Press, 191-218,doi.org/10.1016/B978-0-323-90910-5.00008-7.
- Enin, G.N., Okokon, J.E., Onukak, J.S. (2021). Phytochemical Screening of Solenostemon monostachyus and the Effect of Extract and Fractions on Castor Oil-Induced Diarrhoea in Rats. Tropical Journal of Natural Product Research. 5(4):626-629.
- Hasan, K., Ara, I., Mondal, M.S.A., and Kabir, Y. (2021). Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon 7.1-10
- Ullah, A., Munir, S., Badshah, S.L., Khan, N., Ghani, L., Poulson, B.G., Emwas, A.H., and Jaremko, M. (2020). Important Flavonoids and Their Role as a Therapeutic Agent. Molecules. 25(22):5243. doi: 10.3390/molecules25225243.
- Liu, F., Li, L., Tian, X., Zhang, D., Sun, W., and Jiang, S. (2021). Chemical Constituents and Pharmacological Activities of Steroid Saponins Isolated from Rhizoma Paridis. Journal of Chemistry. Article ID 1442906, 7 pages https://doi.org/10.1155/2021/1442906.
- Bland, J.S. (2021). Application of Phytochemicals in Immune Disorders: Their Roles Beyond Antioxidants. Integrated Medicine (Encinitas). 20(5):16-21.
- Mondal, A., Gandhi, A., Fimognari, C., Atanasov, A.G., Bishayee, A. .(2019). Alkaloids for cancer prevention and therapy: Current progress and future perspectives. European Journal of Pharmacology. 858, https://doi.org/10.1016/j.ejphar.2019.172472.
- Liang, S., and Liu, L. (2019). Sparteine exerts anticancer effect on human cervical cancer cells via induction of apoptosis, G0/G1 cell cycle arrest and inhibition of VEGFR2 signalling pathway. Tropical Journal of Pharmaceutical Research, 18(7), 1455–1460.
- Li, X.X, Liu, C., Dong, S.I., Ou, C.S., Lu, J.L, Ye, J.H., Liang, Y.R., Zheng, X.Q. (2022). Anticarcinogenic potentials of tea catechins. Frontiers in Nutrition. doi.10.3389/fnut.2022.1060783.
- Imran, M., Salehi, B., Sharifi-Rad, J., Aslam Gondal, T., Saeed, F., Imran, A., Shahbaz, M., Tsouh Fokou, P.V., Umair Arshad, M., Khan, H., Guerreiro, S.G., Martins, N., and Estevinho, L.M. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules. 19;24(12):2277. doi: 10.3390/molecules24122277.
- Almatroudi A., Allemailem K.S., Alwanian W.M, Alharbi B.F., Khan A.A., Almatroodi S.A., Rahmani A.H. (2023) . Effect and MechaniS. monostachyus of Kaempterol in the management of Cancer through modulation of inflammation and signal transduction pathway. International Journal of. Molecular Science. 24(10) 8630
- Vilakazi, H., Olasehinde, T.A, and Olaniran, A.O. (2021). Chemical Characterization, Antiproliferative and Antioxidant Activities of Polyunsaturated Fatty Acid-Rich Extracts from Chlorella sp. S14. Molecules. 6;26(14):4109. doi: 10.3390/molecules26144109.
- Deng B, Kong W, Suo H, Shen X, Newton MA, Burkett WC, Zhao Z, John C, Sun W, Zhang X, Fan Y, Hao T, Zhou C, and Bae-Jump VL. (2023). Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer. Cancers (Basel). 14;15(22):5407. doi: 10.3390/cancers15225407.
- Sohrabi, Y., Mohammadzadeh-Aghdash, H., Baghbani, E., Dehghan, P., and Ezzati Nazhad Dolatabadi, J. (2018). Cytotoxicity and Genotoxicity AssesS. monostachyusent of Ascorbyl Palmitate (AP) Food Additive. Advanced Pharmaceutical Bulletin. 8(2):341-346. doi: 10.15171/apb.2018.039.
- Song, J., Wang, Y., Fan, X., Wu, H., Han, J., Yang, M., Lu, L., and Nie G. (2019). Trans-vaccenic acid inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via a mitochondrial-mediated apoptosis pathway. Lipids Health and Diseases. 18:1–9. doi: 10.1186/s12944-019-0993-8.
- Chan, I.S., Knútsdóttir, H., Ramakrishnan, G., Padmanaban, V., Warrier, M., Ramirez, J.C., Dunworth, M., Zhang, H., Jaffee, E.M., Bader, J.S., and Ewald, A.J. (2020). Cancer cells educate natural killer cells to a metastasis-promoting cell state. Journal of Cell Biology. 7;219(9):e202001134. doi: 10.1083/jcb.202001134.
- Ju, P., Ho, Y., Chen, P., Lee, H., Lai, S., Yang, S., and Yeh, C. (2021). Kaempferol inhibits the cell migration of human hepatocellular carcinoma cells by suppressing MMP‐9 and Akt signaling. Environmental Toxicology, 36(10), 1981–1989.
- Carrillo‐Garmendia, A., Martinez‐Ortiz, C., Canizal‐Garcia, M., González‐Hernández, J. C., Arvizu‐Medrano, S. M., Gracida, J., Madrigal‐Perez, L. A., and Regalado‐Gonzalez, C. (2022). Cytotoxicity of quercetin is related to mitochondrial respiration impairment in Saccharomyces cerevisiae. Yeast, 39 (11–12), 617–628.

 ETFLIN
Notification
ETFLIN
Notification







