In Silico Analysis of Limonoid-Based Antifeedants from Melia volkensii Targeting the Ryanodine Receptor in Spodoptera frugiperda
by Mohamed Said Rajab ★
Academic editor: Samir Chtita
Sciences of Phytochemistry 3(2): 98-104 (2024); https://doi.org/10.58920/sciphy0302256
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
06 Jun 2024
29 Jul 2024
03 Sep 2024
09 Nov 2024
Abstract: Spodoptera frugiperda is an invasive pest causing significant crop losses worldwide. Resistance development and health and environmental concerns associated with synthetic insecticides have prompted a search for eco-friendly biopesticides. Limonoids such as salannin, volkensin, and volkensinone, isolated from the East African plant Melia volkensii, show antifeedant activity against S. frugiperda larvae. Volkensin had an ED50 of 3.5 µg/cm², volkensinone (a lactone of volkensin) an ED50 of 6 µg/cm², and salannin an ED50 of 13 µg/cm². Additional limonoids from M. volkensii, including salanninolide and toosendanin, also displayed strong antifeedant effects. With toosendanin already used commercially, a re-evaluation of M. volkensii antifeedant compounds was conducted using in silico techniques. Docking simulations with 3D models of these limonoids and the S. frugiperda ryanodine receptor protein revealed binding affinities from -6.4 to -7.5 kcal/mol, comparable to those of chlorantraniliprole, a commercial insecticide targeting ryanodine receptors. These binding affinities at two distinct receptor sites align well with in-vitro antifeedant activity, underscoring M. volkensii’s potential for environmentally friendly, receptor-targeted biopesticide development against S. frugiperda.
Keywords: Molecular dockingVolkensinSalannin
Introduction
The Fall Armyworm (Spodoptera frugiperda, J. E. Smith) is a highly destructive and polyphagous insect pest that damages economically important crops worldwide (1). Known for its ability to adapt to different environments, this invasive pest frequently causes large outbreaks due to its high feeding rate and versatility in diet (2). While it feeds on a variety of plants, maize and rice are among its primary hosts (3). Its high fecundity, extended adult life span, and rapid reproduction rate contribute to its significant impact on crops (4). Presently, S. frugiperda has spread to over 44 countries in sub-Saharan Africa, causing extensive damage that, according to the International Centre for Agriculture and Biosciences, has exceeded $6 billion (5, 6). In maize alone, infestations can lead to yield losses ranging from 15% to 73% (3).
Due to the severe impact of S. frugiperda infestations, there has been an increased reliance on insecticide spraying. However, repeated and widespread insecticide use has led to S. frugiperda populations developing resistance to various insecticide classes, including benzoylureas, organophosphates, pyrethroids, and carbamates (7-11). This excessive use also has detrimental effects on soil health, human health, and the environment (12-14). The synthetic diamide insecticide chlorantraniliprole (see Figure 1) is commonly used to control FAW, but its continued application has led to resistance in multiple lepidopteran pests, including FAW (15). Chlorantraniliprole targets the ryanodine receptor (RyR) in insects, which disrupts muscle function, leading to paralysis and death (16). The RyR, an intracellular calcium channel essential for insect muscle contraction, is therefore a key target of diamide insecticides. Although diamides are highly effective against RyR, the rise of resistance has driven interest in discovering new types of RyR-targeted compounds.
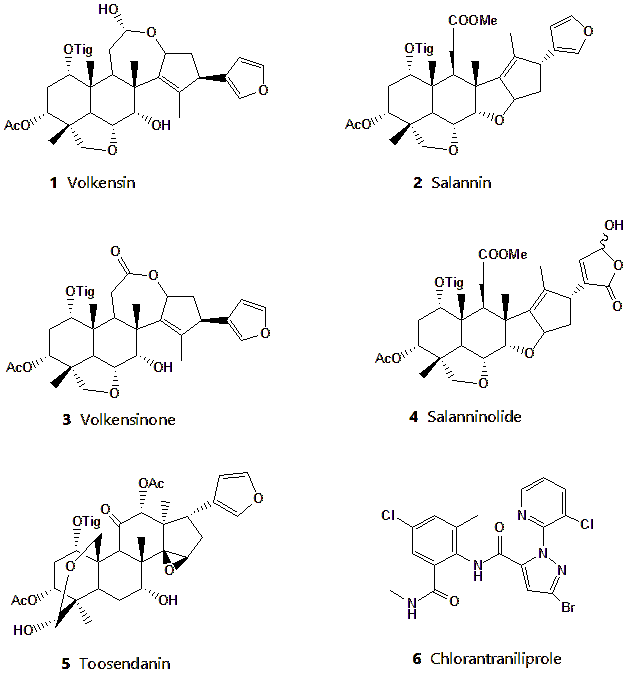
Figure 1. Structures of limonoids volkensin (1), salannin (2), volkensinone (3) salanninolide (4), toosendanin (5) and the commercial insecticide chlorantraniliprole (6).
Research on plant-based biopesticides has shown promise for eco-friendly pest control, as these biopesticides are generally less toxic to non-target organisms and are biodegradable (19). A variety of plant-derived compounds, particularly those with a limonoid structure, have shown antifeedant, insecticidal, and growth-regulating effects against FAW (20-24). Toosendanin (Compound 5, Figure 1), a limonoid-based commercial biopesticide, is one such compound that disrupts digestive and detoxification enzymes in FAW larval midguts (25).
Our previous studies identified three C-seco limonoids, which are volkensin, salannin, and volkensinone, from Melia volkensii with notable antifeedant effects against FAW (26, 27). More recent studies also identified limonoids such as salanninolide and toosendanin from M. volkensii, which have strong antifeedant properties (28, 29). These findings prompted us to re-evaluate our earlier data in light of new results using in silico techniques. This study aimed to evaluate the binding affinities of selected limonoids from M. volkensii, including volkensin, salannin, volkensinone, salanninolide, and toosendanin, against the RyR protein of FAW. We compared these findings to chlorantraniliprole, a commercial diamide insecticide known to target FAW RyR.
Experimental Section
In Vitro Bioassay
The compounds were evaluated for antifeedant activity in choice assays against larvae of the fall armyworm, S. frugiperda. Chemicals dissolved in acetone were evenly distributed on the upper surface of 1 cm2 corn-leaf disks, and the acetone was allowed to evaporate. Control disks received only acetone. Five treated and five untreated disks were alternately pinned in a 9 cm petri dish arena. A 1-day old third instar was placed in the dish, and the assay was conducted at 27o for 15 h with 10 arenas per treatment. Assays were conducted at 10, 3,2 and 1 µg/cm2. Amount of leaf material consumed was determined by weighing the oven-dried remains of disks for each assay and subtracting this from a mean initial weight obtained by drying additional disks. Percent of feeding reduction (% FR) was determined by the equation:
Equation 1
These values were used to determined effective dosages for reduction in feeding by 50% (ED50) (26).
Molecular Docking
Molecular docking was performed using AutoDock Vina embedded in PyRx 0.8. The ryanodine receptor protein of S. frugiperda (FAW) was downloaded from UniProtKB (Accession Reference AOA410JAL6) and used as the receptor protein (30, 31). Five selected limonoids from M. volkensii served as ligands, while the synthetic diamide insecticide chlorantraniliprole, known for its activity against FAW, was used as a reference ligand (32). The docking grid box was positioned to cover the entire RyR protein surface, allowing exploration of potential binding sites distinct from the established binding site for chlorantraniliprole. Since insect ryanodine receptors (RyRs) display multiple binding domains, including diamide, ryanoid, caffeine, and ATP sites, identifying novel chemotypes that bind uniquely within the RyR could bypass resistance associated with specific site mutations (17, 18).
Ligand Retrieval and Preparation
Five limonoids from M. volkensii, including volkensin (CID 6438338), salannin (CID 6437066), volkensinone, salanninolide (CID 76309326), and toosendanin (CID 9851101), were chosen based on their reported antifeedant properties against FAW (26, 28, 29). Chlorantraniliprole was included as a reference to compare with the limonoid antifeedants. Structures for the compounds were retrieved from the PubChem Database in SDF format and converted to MOL2 format using Open Babel version 2.3.1 (33). Structures were then energy minimized with Open Babel using the MMFF94 force field, a steepest descent algorithm with 500 steps, and a convergence parameter of 10e-7. Finalized 3D structures were prepared in PDBQT format for docking with AutoDock tools 1.5.7.
Receptor Preparation and Molecular Docking
Since no 3D structure of FAW's ryanodine receptor is available in the Protein Data Bank, a predicted 3D structure was obtained from UniProtKB (accessed May 24, 2024). The receptor's structure was validated through the SAVES V6.9-Structure Validation Server, achieving a 97.02% validation score, indicating high quality. Preparation steps involved using UCSF ChimeraX for removal of heteroatoms, adding hydrogens, and assigning charges. Docking was conducted with AutoDock Vina, using PyRx 0.8 for the FAW ryanodine receptor and each ligand. Visualization of binding interactions was performed with PyMOL, Protein Plus, and Discovery Studio Visualizer version 2020. The search space parameters included center coordinates (x = -9.0067, y = -1.8424, z = -4.3339) and dimensions (x = 125.4481, y = 110.8430, z = 127.1175 Å) with an exhaustiveness setting of 8. Docking results were analyzed based on RMSD clustering, ΔG binding free energy, and interaction with active site residues. Binding postures were visualized with Ligplot Plus, Protein Plus, and Discovery Visualizer.
Prediction of Insecticide Potency (Tice Rule)
For insecticide development, bioavailability, bioactivity, and toxicity are critical parameters. The insecticide-likeness of the M. volkensii limonoids was evaluated using Tice rule criteria, which specify that effective insecticides should have a molecular weight ≤500 g/mol, hydrogen-bond donors ≤3, hydrogen-bond acceptors ≤12, partition coefficient (log P) ≤5, and rotatable bonds ≤12 (34, 35). The molecular properties of each limonoid were calculated with Molinspiration Cheminformatics online tools (https://www.molinspiration.com).
Results and Discussion
Molecular Docking
In this study, five limonoids from M. volkensii with known antifeedant potential were assessed through molecular docking against the ryanodine receptor protein of FAW. Their docking behavior was compared to that of the commercial insecticide chlorantraniliprole. The docking scores, representing binding affinities, for the limonoids were as follows: volkensin (-7.5 kcal/mol), salannin (-6.4 kcal/mol), volkensinone (-7.5 kcal/mol), salanninolide (-6.8 kcal/mol), toosendanin (-7.5 kcal/mol), and chlorantraniliprole (-6.7 kcal/mol) as shown in Table 1. These compounds (except salannin) demonstrated comparable binding affinities and interacted with different amino acids within two distinct binding sites on the FAW ryanodine receptor.
Toosendanin and chlorantraniliprole were observed to bind to a known site on the FAW ryanodine receptor, as reported in earlier studies (16, 17). Toosendanin formed hydrogen bonds with Pro172, Lys178, and Thr206, and carbon-hydrogen bonds with Gly202 and Lys182. Chlorantraniliprole bonded with Met200, Asn199, and Lys163 via hydrogen bonds and with Leu164 through a pi-alkyl bond. Meanwhile, volkensin, salannin, volkensinone, and salanninolide bound at a different site on the receptor (18). For instance, volkensin interacted with Leu47 and Val89, while volkensinone formed bonds with Val90, Asn260, Ser64, and Phe259. Salannin, with the lowest binding affinity, bonded with Tyr246, Val273, and Trp249. Figures 2 and 3 depict these interactions, and Table 1 outlines the binding energies and amino acid interactions.
Table 1. Binding affinities of five limonoid-based antifeedants from Melia volkensii compared to commercial synthetic insecticide chlorantraniliprole.
|
Ligand |
Binding Energy (kcal/mol) |
Predicted dissociation constant Kd* |
Number of the various interactions |
||||
|
Conventional H-Bond |
Alkyl |
Pi- Alkyl |
Carbon -Hydrogen |
Pi- Sigma |
|||
|
Volkensin |
-7.5 |
3.14x10-6M |
0 |
0 |
2 |
0 |
0 |
|
Salannin |
-6.4 |
2.01x10-5M |
1 |
0 |
1 |
0 |
1 |
|
Volkensinone |
-7.5 |
3.14x10-6M |
3 |
0 |
1 |
1 |
0 |
|
Salanninolide |
-6.8 |
1.02x10-5M |
2 |
1 |
0 |
1 |
0 |
|
Toosendanin |
-7.5 |
3.14x10-6M |
3 |
0 |
0 |
2 |
0 |
|
Chlorantraniliprole |
-6.7 |
1.21x10-5M |
3 |
0 |
1 |
0 |
0 |
|
Note: *The dissociation constants Kd is a measure of binding affinity which is used to evaluate and rank the order of strengths of bimolecular interactions of the ligand and the target protein. The smaller the Kd value, the greater the binding affinity of the ligand to the receptor protein. |
|||||||
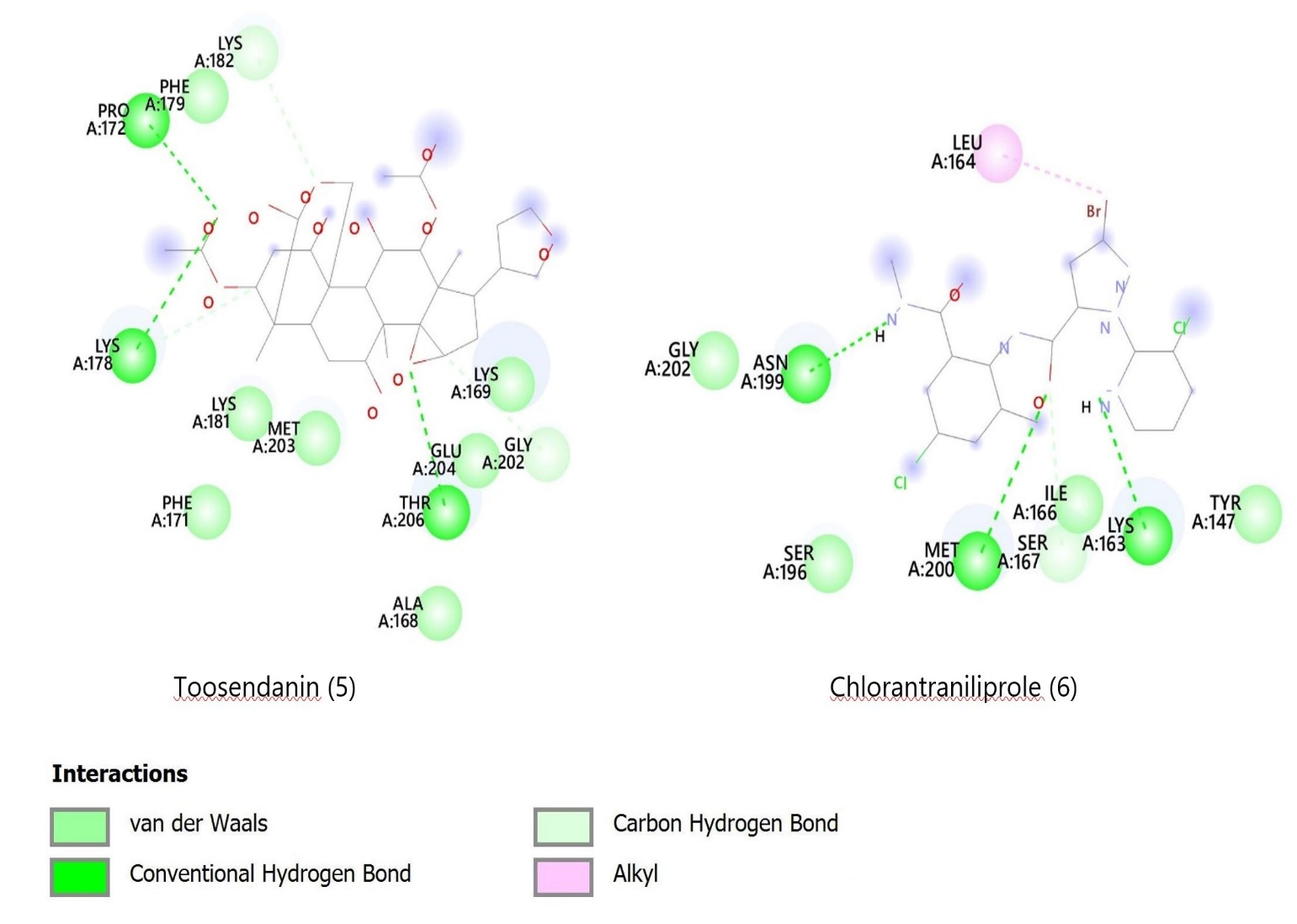
Figure 2. 2D diagrams showing docking interactions of M. volkensii compounds 1- 4 with the target ryanodine receptor protein of the insect FAW.
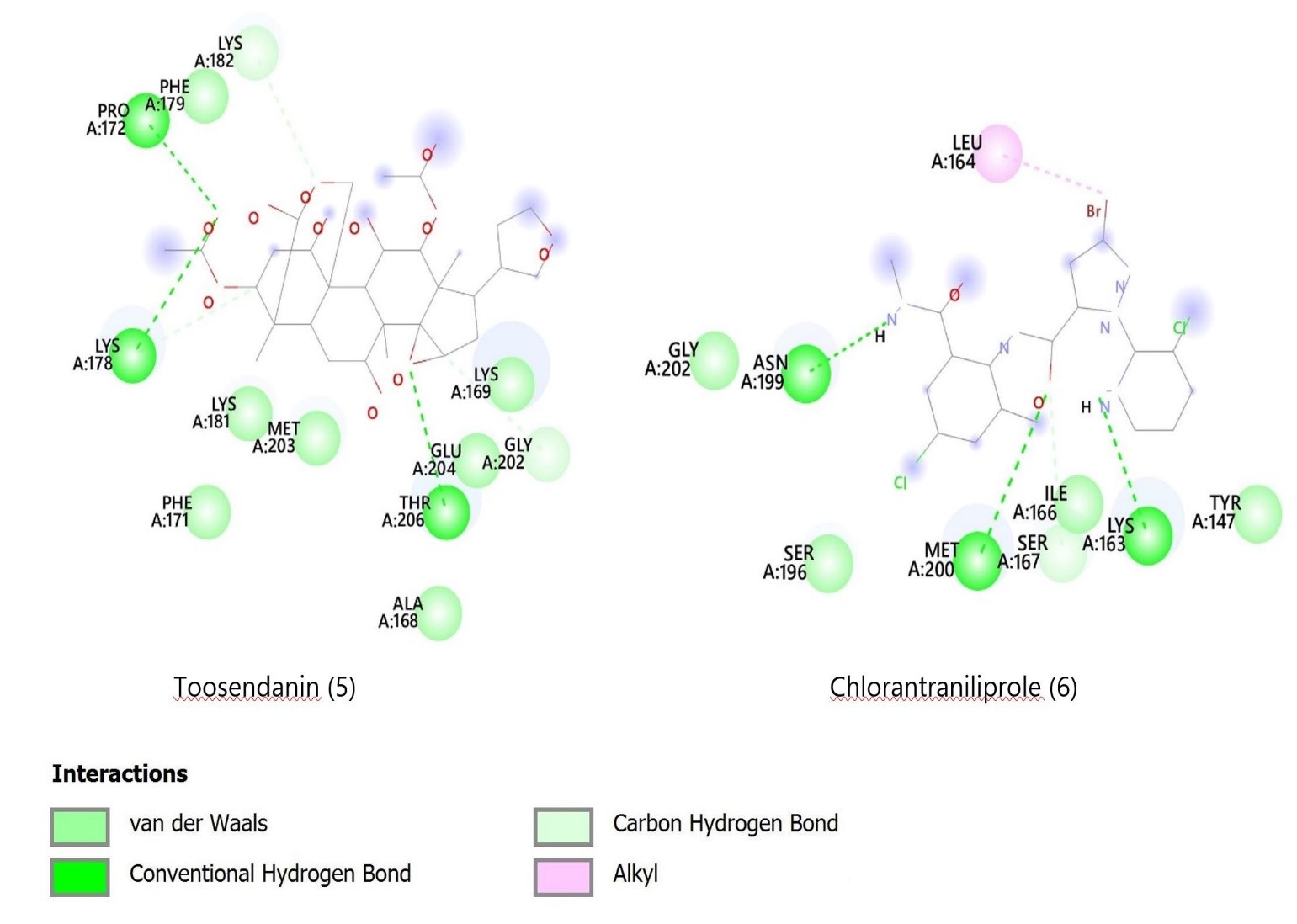
Figure 3. 2D diagrams showing`docking interactions of Melia volkensii compounds 5 and chlorantraniliprole (6) with the target ryanodine receptor protein of the FAW.
The electrostatic and van der Waals interactions between the ryanodine receptor protein and the six ligands are detailed in Table 1 and Figures 2 and 3. The docking results indicate that M. volkensii limonoids volkensin, volkensinone, and toosendanin have significant binding potential with the target ryanodine receptor, showing affinities of -7.5 kcal/mol. The stability of these ligand-protein complexes is influenced by multiple molecular interactions, including hydrogen bonding, pi-alkyl, pi-pi, pi-sigma, and van der Waals interactions. Notably, like the commercial insecticide chlorantraniliprole (6), both volkensinone and toosendanin demonstrated three conventional hydrogen bonds, suggesting that they may possess insecticidal activity comparable to that of chlorantraniliprole against FAW.
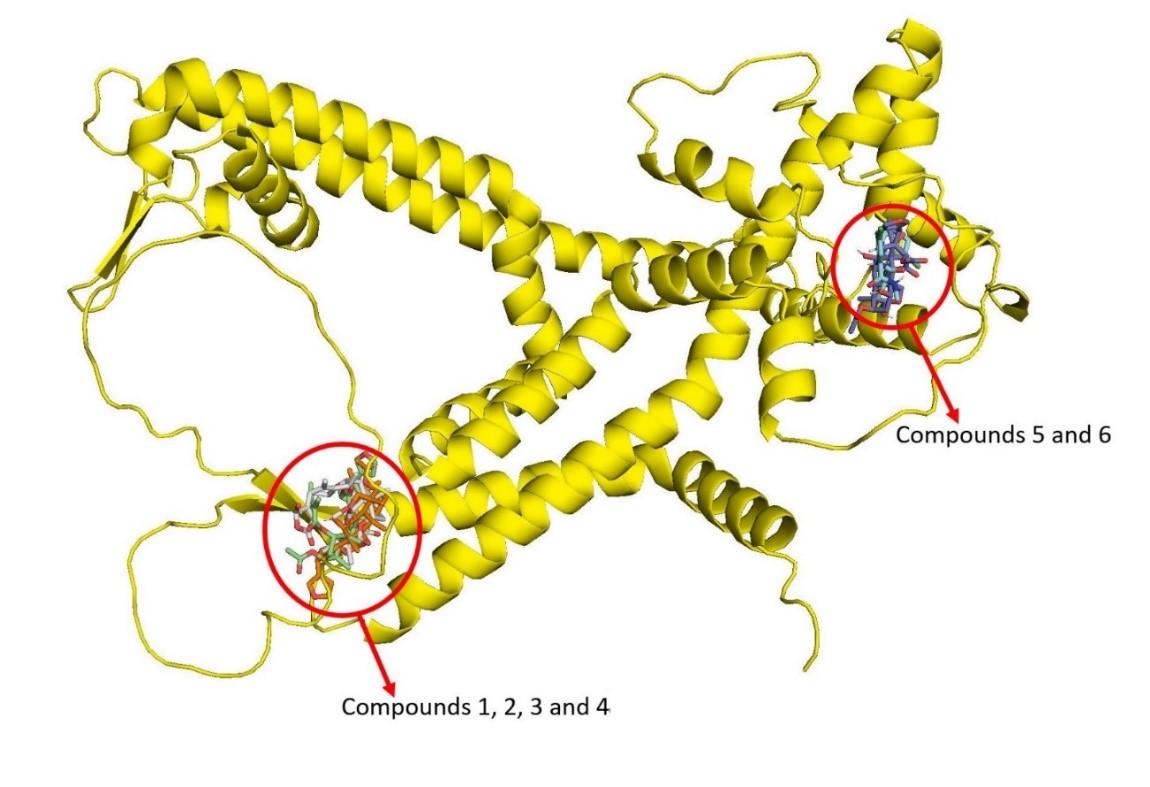
Figure 4. Ryanodine receptor protein showing clustering of the docked compounds 1 – 6 in two groups of compounds 1, 2, 3 and 4 and compounds 5 and 6.
The docking analysis identified two primary binding regions on the ryanodine receptor (Figure 4). Toosendanin and chlorantraniliprole were found to dock at the main receptor site, which is crucial for FAW interactions (16, 17), while the remaining C-seco limonoids bound at a different site (18). The unique binding of volkensin, salannin, volkensinone, and salanninolide to this alternate site may provide an advantage in countering resistance arising from mutations at the primary ryanodine receptor site (18, 36).
Table 2. Tice rule properties of scored Melia volkensii limonoids and chlorantraniliprole.
|
Ligand |
MW |
mi-LogP |
Rule of 5 Violations |
No. of Rotatable Bonds |
H-bond acceptor |
T PSA |
H-bond donor |
|
Volkensin |
584.71 |
4.55 |
1 Mw |
6 |
9 |
124.67 |
2 |
|
Salannin |
596.72 |
5.40 |
2 Mw, mi logP |
9 |
9 |
110.52 |
0 |
|
Volkensinone |
582.69 |
4.78 |
1 Mw |
6 |
9 |
121.52 |
1 |
|
Salanninolide |
628.72 |
4.75 |
2 Mw, NO |
9 |
11 |
143.92 |
1 |
|
Toosendanin |
574.62 |
0.84 |
2 Mw, NO |
5 |
11 |
165.27 |
3 |
|
Chlorantraniliprole |
483.15 |
3.12 |
0 |
4 |
7 |
88.91 |
2 |
Tice Rule and Insecticidal Likelihood
As presented in Table 2, M. volkensii compounds 1 and 3 each violated only the molecular weight parameter, rendering them largely compliant with the Tice rule. However, salannin, salanninolide, and toosendanin violated both the molecular weight and LogP criteria (34, 35). According to the Tice rule, ideal insecticides should ideally have only one parameter violation, as multiple violations could lead to reduced bioavailability or other efficacy issues. Nonetheless, it is noteworthy that not all successful insecticides align strictly with theoretical insecticide-likeness predictions.
Conclusion
The rise of pesticide resistance to synthetic insecticides has increased the importance of natural products in the quest for sustainable and eco-friendly pest control agents. In silico studies demonstrated that the limonoids from M. volkensii, including volkensin, salannin, volkensinone, salanninolide, and toosendanin, exhibited binding affinities to the ryanodine receptor protein ranging from -6.4 kcal/mol to -7.5 kcal/mol. These affinities are comparable to those of the commercial insecticide chlorantraniliprole. Notably, the distinct binding sites of volkensin, salannin, volkensinone, and salanninolide within the RyR protein, in contrast to the binding sites of the commercial insecticides toosendanin and chlorantraniliprole, suggest that this class of C-seco limonoids could serve as potential lead compounds for a new category of chemotypes. These compounds may bind to a site on the RyR that differs from that of diamide insecticides, potentially circumventing resistance associated with RyR mutations.
Moreover, the strong binding affinity of volkensinone, measured at -7.5 kcal/mol and featuring three conventional hydrogen bonds, is similar to that of the commercial botanical insecticide toosendanin. This similarity indicates the need for further in vitro and in vivo studies to explore its potential as a new sustainable botanical insecticide. Additionally, evaluating extracts of M. volkensii that contain a blend of C-seco limonoids, such as volkensin, volkensinone, salanninolide, and toosendanin, may prove beneficial as an eco-friendly biopesticide in integrated pest management strategies for FAW. Such extracts would be affordable, biodegradable, non-toxic, target-specific, and less likely to foster resistance in insect pests.
Declarations
Acknowledgment
The author acknowledges and appreciates the Pwani University Council for allowing him to spend his sabbatical leave as a visiting professor at the Technical University of Mombasa.
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Lima AF, Ribeiro LP, Lira SP, Carvalho GA and Vendramim JD. Growth inhibitory activities and feeding deterrence of solanaceae - based derivatives on fall armyworm. Agriculture 2023; 13: 420.
- Song Z, Li C, Tan Y, Shen S, Gong Y, Wang Y, Wang R, Hernandez Z, Chen J and Zhang Z. Chlorantraniliprole emulsified with botanical oils effectively controls invasive pest Spodoptera frugiperda larvae in corn plant. J Pest Sci. 2023; https://doi.org/10.1007/s10340-023-01628-2.
- Ullah MS, Sharmin D, Tumpa TA, Rashed MT, Mondal P, Akram MW, Chowdhury S, Ahmad M, Gotoh T and Chaudhary M. Invasion, distribution, monitoring and farmers perception of fall armyworm (Spodoptera frugiperda) and farm-level management practices in Bangladesh. Insects 2023; 14: 343.
- Xiao H, Ye X, Xu H, Mei Y, Yang Yi, Chen, Xi, Yang Y, Liu T, Yu Y, Yang W, Lu Z, Li F. The genetic adaptations of fall armyworm Spodoptera frugiperda facilitated its rapid global dispersal and invasion. Mol Ecol Resour. 2020; ;20(4):1050-1068.
- Fotso Kuate A, Hanna R, Doumtsop Fotio ARP, Abang AF, Nanga SN, Ngatat S, et al. (2019) Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Cameroon: Case study on its distribution, damage, pesticide use, genetic differentiation and host plants. PLoS ONE. 2019; 14(4) 1-18.
- Tambo J A, Day R K, Lamontagne-Godwin J, Silvestri S, Beseh P K, Oppong-Mensah B, Noah A, Phiri M M. (2020) Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: an analysis of farmers’ control actions, International Journal of Pest Management, 2020; 66:4, 298-310,
- Chen H-L, Hasnain A, Cheng Q-H, Xia L-J, Cai Y-H, Hu R, Gong C-W, Liu X-M, Pu J, Zhang L and Wang X-G. Resistance monitoring and mechanism in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) for chlorantraniliprole from Sichuan Province, China. Front. Physiol. 2023; 14:1180655.
- Diez-Rodríguez GI, Omoto C (2001) Inheritance of lambdacyhalothrin resistance in Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Neotrop Entomol. 2001; 30:311-316.
- Carvalho RA, Omoto C, Field LM, Williamson MS, Bass C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. Plos One. 2013; 8:e62268.
- Yu S J. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (J. E. Smith) Pesticide Biochemistry and Physiology. 1991; 39, 1:84-91.
- Yu S J, Nguyen S N, Abo-Elghar G.E. Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith). Pesticide Biochemistry and Physiology Volume 77, Issue 1, September 2003, Pages 1-11.
- Tripathi S, Srivastava P, Devi R S, Bhadouria R. Chapter 2 - Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. Agrochemicals Detection, Treatment and Remediation. Pesticides and Chemical Fertilizers 2020; 25-54.
- Grewal, A.S., et al. Pesticide Res-idues in Food Grains, Vegetables and Fruits:A Hazard to Human Health. J Med Chem Toxicol. 2017; 2(1): 1- 7.
- Mahmood I, Imadi S R, Shazadi K, Gul A, and Hakeem K R. Effects of Pesticides on Environment. Plant, Soil and Microbes. 2016; 253 DOI 10.1007/978-3-319-27455-3_13.
- Cordova D, Benner E A, Clark D A, Bolgunas S P, Lahm G P, Gutteridge S, Rhoades D F, Wu L, Sopa J S, Rauh J J, Barry J D. Pyrrole-2 carboxamides - A novel class of insect ryanodine receptor activators. Pesticide Biochemistry and Physiology. 2021; 174.
- Lahm GP, Cordova D, Barry JD. New and selective ryanodine receptor activators for insect control. Bioorg Med Chem. 2009; 15;17(12) :4127-33.
- Ma R, Haji-Ghassemi O, Ma D, Jiang H, Lin L, Yao L, Samurkas A, Li Y, Wang Y, Cao P, Wu S, Zhang Y, Murayama T, Moussian B, Van Petegem F, Yuchi Z. Structural basis for diamide modulation of ryanodine receptor. Nat Chem Biol. 2020; 16(11):1246-1254.
- Ren H, Zhang H, Ni R, Li Y, Li L, Wang W, Tian Y, Pang B, Tan Y. Detection of ryanodine receptor G4911E and I4754M mutation sites and analysis of binding modes of diamide insecticides with RyR on Galeruca daurica (Coleoptera: Chrysomelidae). Front Physiol. 2022; 22;13:1107045.
- Tulashie S K, Francis A, Abraham J, Addo E. Potential of neem extracts as a natural insecticide against fall armyworm (Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae)). Case Studies in Chemical and Environmental Engineering. 2021; 4: 100130.
- Koul O, Singh G, Singh R, Singh J, Daniewski WM and Berlozecki S. (2004) Bio efficacy and mode-of-action of some limonoids of salannin group from Azadirachta indica A. Juss and their role in a multicomponent system against lepidopteran larvae. J. Biosci. 2004; 29(4): 409-416
- Roy A, and Saraf S. Limonoids: Overview of Significant Bioactive Triterpenes Distributed in Plants Kingdom. Biol. Pharm. Bull. 2006; 29(2): 191—201.
- Paredes-Sánchez FA, Rivera G, Bocanegra-García V, Martínez-Padrón HY, Berrones-Morales M, Niño-García N, Herrera-Mayorga V. Advances in Control Strategies against Spodoptera frugiperda. A Review. Molecules. 2021; 15, 26(18):5587.
- Fan W, Fan L, Wang Z and Yang L (2022) Limonoids From the Genus Melia (Meliaceae): Phytochemistry, Synthesis, Bioactivities, Pharmacokinetics, and Toxicology. Front. Pharmacol. 12:795565.
- Mendel M J, Alford A R, Rajab M S and Bentley M D. Relationship of Citrus Limonoid Structure to Feeding Deterrence Against Fall Armyworm (Lepidoptera: Noctuidae) Larvae. Environ. Entomol. 1993; 22(1)1: 167-173.
- Lin Y, Huang Y, Liu J, Liu L, Cai X, Lin J, Shu B. Characterization of the physiological, histopathological, and gene expression alterations in Spodoptera frugiperda larval midguts affected by toosendanin exposure. Pesticide Biochemistry and Physiology. 2023; 195,105537.
- Rajab M S, Bentley M D, Alford A R and Mendel M J. A new limonoid insect antifeedant from the fruit of Melia volkensii. Journal of Natural Products 1988; 51(1): 168-171.
- Rajab M S and Bentley M D. Tetranortriterpenes from Melia volkensii. Journal of Natural Products 1988; 51(3): 840-844
- Jaoko V, Taning C N T, Backx S, Mulatya J, Abeele J V D, Magomere T, Olubayo F, Mangelinckx S, Stefaan P.O. Werbrouck S P O and Smagghe G. The Phytochemical Composition of Melia volkensii and Its Potential for Insect Pest Management. Plants 2020; 9(2)
- Jaoko V, Taning C.N.T, Backx S, Motti P, Mulatya J, Vandenabeele J, Magomere T, Olubayo F, Mangelinckx S, Werbrouck S P O, et al. Laboratory and Greenhouse Evaluation of Melia volkensii Extracts for Potency againstAfrican Sweet Potato Weevil, Cylas puncticollis, and Fall Armyworm, Spodoptera frugiperda. Agronomy 2021; 11, 1994.
- Sezhian U G and Sekar S. Computational Studies of Potential Insecticidal Limonoids from Neem Against Spodoptera frugiperda (J. E. Smith) International Journal of Zoological Investigations. International Journal of Zoological Investigations. 2023; 9(2):566-574.
- Samurkas A, Yao L, Hadiatullah H, Ma R, Xie Y, Sundarraj R, Zuilhof H and Yuchi Z. (2021) Ryanodine receptor as insecticide target. Curr Pharmaceut Design. 2021; 27:1-10.
- Hasnain A, Zhang S, Chen Q, Xia L, Wu Y, Gong C, Liu X, Jian P, Zhang L, Wang X. Effects of chlorantraniliprole on the life history traits of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Front Physiol. 2023 Mar 30;14: 1155455.
- O'Boyle, N.M., Banck, M., James, C.A. et al. Open Babel: An open chemical toolbox. J Cheminform 2011; 33 (3): 1-14. https://doi.org/10.1186/1758-2946-3-33.
- Tice, CM. Selecting the right compounds for screening: Does Lipinski’s Rule of 5 for pharmaceuticals apply to agrochemicals. Pest Manag Sci. 2001; 57: 3–16.
- Avram S, Funar-Timofei S, Borota A, Chennamaneni S R, Manchala A K and Muresan S. Quantitative estimation of pesticide-likeness for agrochemical discovery Journal of Cheminformatics. 2014; 6(42), 2-11.
- Samurkas A, Fan X, Ma D, Sundarraj R, Lin L, Yao L, Ma R, Jiang H, Cao P, Gao Q, and Yuchi Z. Discovery of Potential Species-Specific Green Insecticides Targeting the Lepidopteran Ryanodine Receptor. Journal of Agricultural and Food Chemistry, 2020; 68 (15): 4528-4537.

 ETFLIN
Notification
ETFLIN
Notification







