Phytochemistry and GCMS Analysis of Ethanol and Aqueous Stembark Extracts of Detarium microcarpum Guill. & Perr. Fabaceae
by Mubarak Muhammad Dahiru ★ , Neksumi Musa, Enoch Buba Badgal
Academic editor: Samir Chtita
Sciences of Phytochemistry 4(1): 9-19 (2025); https://doi.org/10.58920/sciphy0401268
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
02 Aug 2024
01 Dec 2024
17 Dec 2024
09 Jan 2025
Abstract: The therapeutic applications of medicinal plants in the treatment of various diseases can be attributed to their diverse phytochemical constituents. This study aimed to investigate the phytochemical composition of aqueous and ethanol stem bark extracts of Detarium microcarpum. Qualitative and quantitative analyses were conducted to determine the presence and concentrations of phytochemicals, followed by the identification of phytoconstituents using gas chromatography-mass spectrometry (GC-MS). The aqueous extract was found to contain saponins (27.11 ± 0.22%) and flavonoids (47.33 ± 0.70% ), with alkaloids, steroids, glycosides, and terpenoids absent. In contrast, the ethanol extract contained alkaloids (10.78 ± 0.59%), saponins (45.11 ± 0.48%), glycosides (5.44 ± 0.48%), and flavonoids (11.00 ± 0.77%), while steroids and terpenoids were not detected. GC-MS analysis revealed 14 compounds in the aqueous extract and 20 in the ethanol extract. The major constituents of the aqueous extract included hydroperoxide, 1,4-dioxan-2-yl (58.32%), 1,2,3-benzenetriol (16.44%), and cis-p-coumaric acid (11.05%). In the ethanol extract, the predominant compounds were coumarin (29.41%), benzofuran (17.23%), and catechol (9.23%). The identified compounds may serve as potential synthetic templates for the development of novel therapeutic agents targeting various diseases. This study supports the ethnomedicinal use of D. microcarpum and provides a scientific basis for its role in traditional medical practices.
Keywords: EthnomedicinePhytotherapyProfilingCoumarinCatechol
Introduction
The phytochemical composition of medicinal plants has been the reason for their extensive employment in ethnomedicine. These phytochemicals constitute different bioactive compounds that are attributed to the pharmacological and biological activities of the different plant parts. In traditional practice, different parts of plants are prepared in different formulations; maceration, decoctions, and infusions, administered to achieve therapeutic goals (1). Medicinal plants exhibit efficacy in phytotherapy of various ailments using different plant parts (2-7). Poor and ineffective medical care and the cost of drugs especially in developing countries have been the driving force for the prospect of medicinal plants, thus affordable alternative (8). Over 100,000 different kinds of plants are associated with various pharmacological and biological activities attributed to the phytoconstituents of the plants (9-12).
Detarium microcarpum (D. microcarpum) tree is native to Africa and grows in Central and West African regions (13). The plants are called tallow three in English and Taura by the native Hausa people in Nigeria. This plant is often applied in ethnomedicine to treat diseases due to the pharmacological activities associated with the plant including the phytotherapy of diarrhea, meningitis, tuberculosis, hemorrhoids, and diabetes (14, 15). D. microcarpum comprises high phenolic compounds with free radical scavenging capabilities, acting as an antioxidant for oxidative stress-linked diseases (16). The antimicrobial activity of D. microcarpum against Streptococcus aureus and Escherichia coli was attributed to their phytochemical composition (17, 18). In another report, the antibacterial activity of D. microcarpum against salmonella thyphimurium supported its application in the phytotherapy of typhoid infections (19). Antimalarial activity of D. microcarpum was previously reported in infected mice and an assessment of the hematological and biochemical parameters revealed the safety of the plants (20).
A previous study reported various pharmacological activities of different parts of D. microcarpum, including hypoglycemic, antimicrobial, anti-parasitic, and anti-inflammatory properties (15, 18). Furthermore, the plant was reported to be a source of novel drugs attributed to its biological activities of the plant against Alzheimer's and snake venoms (21). In another study, D. microcarpum was reported to possess anticancer activity evident to its cytotoxicity which was linked to its application in ethnomedicine and potential for the development of novel anticancer drugs (22). Moreover, it has been traditionally employed in diabetic therapy in most regions of Africa (23). An ethnobotanical survey of traditionally used plants for the management of cancer reported the use of the root and stembark of D. microcarpum in Borno State, Nigeria (24). The plant of study has been reported to have different pharmacological activities, attributed to their phytochemical composition (25). Thus, this study aimed to examine the phytochemical profile of the aqueous and ethanol stembark extracts of D. microcarpum due to its extensive use in traditional ethnomedicine.
Methodology
Materials
The stembark of D. microcarpum was obtained from Girei Local Government Area of Adamawa state, Nigeria. It was authenticated by a Forest Technologist from the Forestry Department, Adamawa State Polytechnic Yola, where a voucher specimen with voucher number ASP/FT/0099 was deposited. The stembark was air-dried and ground into powder using a blender.
Extract Preparation
D. microcarpum stembark powder (300 g) was macerated in 1 L of distilled water and ethanol separately in glass jars for 48 h. The mixtures were separately filtered using a Whatman no. 1 filter paper, followed by concentrating the filtrate to dryness in an oven at 40 °C to yield 21 and 34 g of aqueous (ASBE) and ethanol (ESBE) stembark of extracts D. microcarpum, respectively (26).
Phytochemical Screening and Quantitation
Phytochemical identification of the ASBE and ESBE was carried out by the methods described previously by Evans (26) to detect the presence of alkaloids, saponins, steroids, glycosides, terpenoids, and flavonoids.
Total Alkaloids
Alkaloids were separately quantified from each extract as previously described by Harborne (27). Exactly 10 mL of 10% ammonium hydroxide was added to 0.2 g of the ASBE and ESBE, separately to convert alkaloidal salts into free bases, followed by stirring. The mixtures were filtered after 4 h and concentrated over a water bath, followed by a drop-wise addition of concentrated ammonium hydroxide to precipitate the alkaloids. The precipitate was filtered using an initially weighted filter paper and washed with 10% ammonium hydroxide solution. The filter paper was dried at 60 °C for 30 min and reweighed to a constant weight. The total alkaloids were determined using Equation 1. All procedures were carried out in triplicates and presented as a mean of triplicate determinations.
Equation 1
Total Saponins
Saponins were separately quantified by the method previously described by Obadoni et al., (28). Exactly 10 mL of 20% aqueous ethanol was added to 0.2 g of the ASBE and ESBE separately, followed by heating and stirring over a water bath at 55 °C for 1 h. The mixtures were transferred to separating funnels, followed by the addition of 5 mL of diethyl ether and vigorous shaking for proper partitioning. The aqueous layer was collected, followed by the addition of 10 mL of n-butanol and 2 mL of 5% NaCl. The mixtures were dried in an oven at 60 °C. The total saponins were determined from Equation 1. All procedures were carried out in triplicates and presented as a mean of triplicate determinations.
Total Glycosides
Glycosides were quantified as described previously by Ugwoke et al., (29). Exactly 10 mL of 70% aqueous ethanol was added to 0.2 g of the ASBE and ESBE, separately and boiled for 2 min in a water bath, followed by filtration. The filtrates were diluted with 20 mL of distilled water, followed by the addition of 2 mL of 10% lead acetate and filtration. Furthermore, 10 mL of chloroform was added to the filtrates in a separating funnel and vigorously shaken. The chloroform layer was collected, dried, and weighed. The total glycosides were determined from Equation 1. All procedures were carried out in triplicates and presented as a mean of triplicate determinations.
Total Flavonoids
Flavonoids were quantified as previously described by Harborne (27). Exactly 10 mL of 80% aqueous methanol was added to 0.2 g of the ASBE and ESBE, separately. The whole solution was filtered and evaporated to dryness over a water bath and weighed. The total flavonoids were determined from Equation 1. All procedures were carried out in triplicates and presented as a mean of triplicate determinations.
Gas
Chromatography-Mass Spectrometric
(GCMS) Analysis
The identification of the compounds in the ASBE and ESBE was done with a gas-chromatography mass spectrophotometer system (Model 7890-5975, Agilent, USA) fitted with a fused silica column. The settings and procedure were as previously described (30-33, 12).
Statistical Analysis
The data obtained were expressed as ± standard error of the mean (± SEM) of triplicate determinations. The data were statistically evaluated using Statistical Package for the Social Sciences (SPSS) software (version 22).
Result
Phytochemical Screening and Quantitation
The phytochemicals detected in the ASBE and ESBE of D. microcarpum are depicted in Table 1. Although alkaloids and glycosides were absent in the ASBE, they were detected in the ESBE. However, both saponins and flavonoids were detected in both extracts. Saponins and flavonoids were detected in the ASBE in concentrations of 27.11 ± 0.22% and 47.33 ± 0.70%, respectively. In the ESBE, saponins had the highest concentration (45.11 ± 0.48%), followed by flavonoids (11.00 ± 0.77%) and alkaloids (10.78 ± 0.59%) while glycosides had the least concentration (5.44 ± 0.48%).
Table 1. Phytochemical composition of the ASBE and ESBE of D. microcarpum.
|
Phytochemical |
ASBE |
ESBE |
|
Alkaloids |
- |
10.78 ±0.59 |
|
Saponins |
27.11 ±0.22 |
45.11 ±0.48 |
|
Steroids |
- |
- |
|
Glycosides |
- |
5.44 ±0.48 |
|
Terpenoids |
- |
- |
|
Flavonoids |
47.33 ±0.70 |
11.00 ±0.77 |
|
Note: Negative (-) means absent |
||
GCMS Analysis
The phytochemical analysis of the aqueous stem bark extract (ASBE) of D. microcarpum identified a total of 14 distinct compounds, as shown in Table 2. Among these, 1,4-dioxanyl hydroperoxide was the most abundant, comprising 58.32% of the extract, followed by 1,2,3-benzenetriol at 16.44%, and cis-p-coumaric acid at 11.05%. Other notable compounds included coumarin, with a peak area of 5.26%, and catechol, contributing 1.99% of the total composition. The chemical structures of the identified compounds are presented in Figure 1, highlighting the functional groups and structural features characteristic of each compound. The chromatographic profile of the ASBE, displayed in Figure 2, provides a detailed visualization of the retention times and peak areas of the identified compounds. The chromatogram illustrates the prominence of 1,4-dioxanyl hydroperoxide as the major peak, along with secondary peaks corresponding to 1,2,3-benzenetriol and cis-p-coumaric acid. These data underscore the chemical complexity of the ASBE and the dominance of specific bioactive components within the extract.
Table 2. Compounds Identified in the ASBE of D. microcarpum.
|
S/N |
Name of compound |
Retention Time |
Peak Area (%) |
Molecular weight |
Formula |
|
1 |
Catechol |
3.99 |
1.99 |
110.11 |
C6H6O2 |
|
2 |
Coumarin |
4.68 |
5.26 |
146.15 |
C9H6O2 |
|
3 |
Cis-p-Coumaric acid |
5.43 |
11.05 |
164.16 |
C9H8O3 |
|
4 |
1,2,3-Benzenetriol |
5.75 |
16.44 |
126.11 |
C6H6O3 |
|
5 |
1,2,4-Benzenetriol |
6.81 |
1.01 |
126.11 |
C6H6O3 |
|
6 |
N-Serylserine |
6.99 |
2.27 |
192.17 |
C6H12N2O5 |
|
7 |
7.82 |
58.32 |
120.11 |
C4H8O4 |
|
|
8 |
Erucic acid |
10.74 |
1.07 |
338.57 |
C22H42O2 |
|
9 |
Trans-13-Octadecenoic acid |
11.91 |
0.50 |
282.47 |
C18H34O2 |
|
10 |
cis-11-Hexadecenal |
12.30 |
0.07 |
238.41 |
C16H30O |
|
11 |
cis-Vaccenic acid |
14.05 |
0.52 |
282.47 |
C18H34O2 |
|
12 |
Oleic Acid |
14.52 |
0.47 |
282.47 |
C18H34O2 |
|
13 |
Petroselinic acid |
14.74 |
0.25 |
282.47 |
C18H34O2 |
|
14 |
Tetradecanal |
15.64 |
0.77 |
212.38 |
C14H28O |
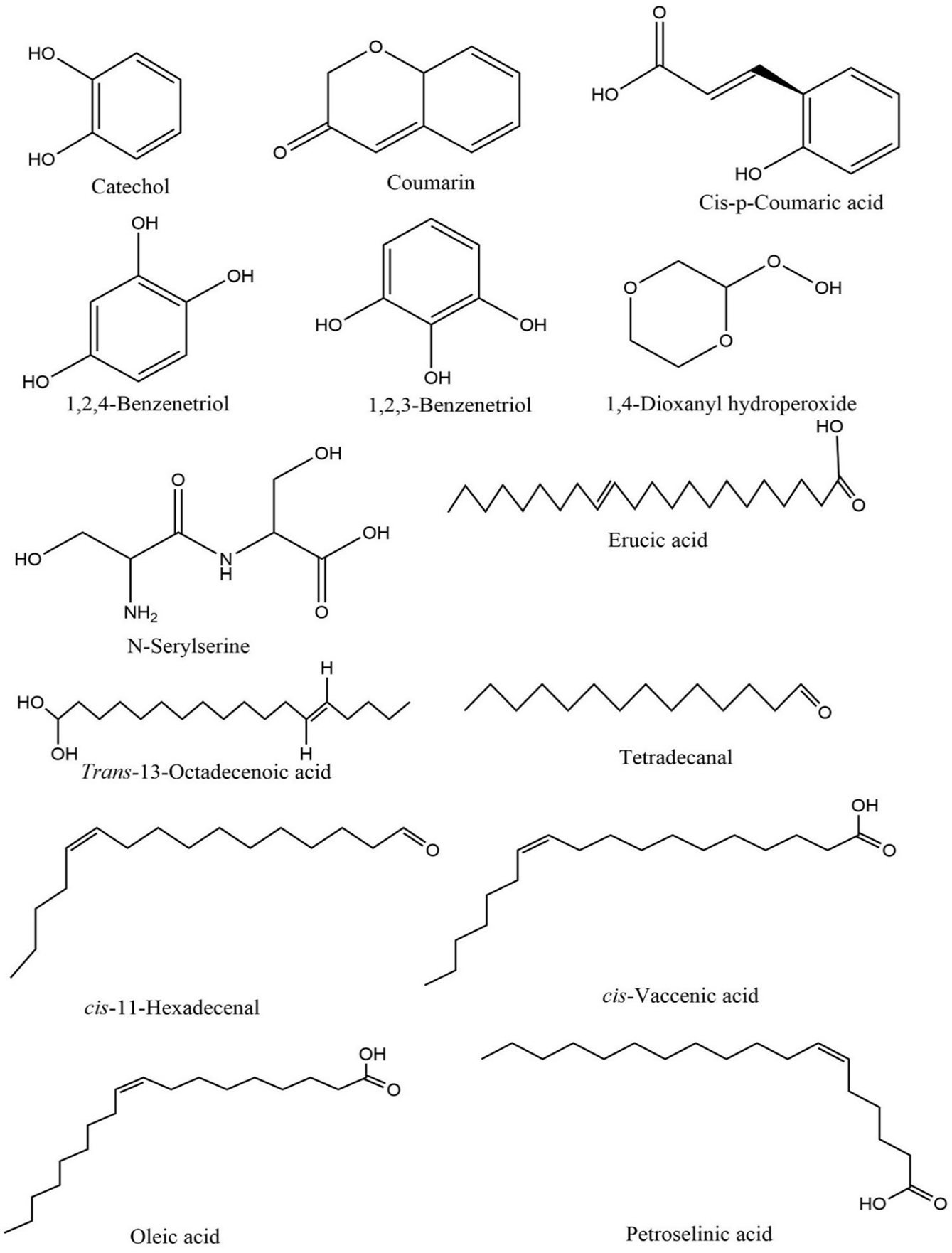
Figure 1. Structures of compounds identified in ASBE of D. microcarpum.
Table 3. Compounds Identified in the ESBE of D. microcarpum.
|
S/N |
Compound |
Retention Time |
Peak Area (%) |
Molecular weight |
Formula |
|
1 |
Catechol |
4.019 |
9.23 |
110.11244 |
C6H6O2 |
|
2 |
Coumarin |
4.620 |
29.41 |
146.14544 |
C9H6O2 |
|
3 |
Benzofuran |
5.919 |
17.23 |
118.13504 |
C8H6O |
|
4 |
Methyl palmitate |
6.978 |
8.59 |
270.45576 |
C17H34O2 |
|
5 |
7.493 |
4.76 |
126.11184 |
C6H6O3 |
|
|
6 |
7.807 |
5.86 |
126.11484 |
C5H6N2O2 |
|
|
7 |
8.431 |
3.87 |
126.11184 |
C6H6O3 |
|
|
8 |
1,3,5-Benzenetriol |
8.614 |
2.57 |
126.11184 |
C6H6O3 |
|
9 |
Phenol, 2-[(1-methylpropyl)thio]- |
8.929 |
2.24 |
182.28056 |
C10H14OS |
|
10 |
cis-Vaccenic acid |
9.129 |
1.82 |
282.46676 |
C18H34O2 |
|
11 |
Methyl cis-7-hexadecenoate |
10.033 |
0.49 |
268.43988 |
C17H32O2 |
|
12 |
2,6,6-trimethylnorpinane |
10.256 |
0.32 |
138.25292 |
C10H18 |
|
13 |
Octadecanal |
10.760 |
0.23 |
268.48324 |
C18H36O |
|
14 |
2,6,10-Trimethylundeca-1,5,9-triene |
11.933 |
1.01 |
192.34456 |
C14H24 |
|
15 |
Trans-Farnesol |
12.328 |
0.23 |
222.37084 |
C15H26O |
|
16 |
9-Tetradecenal, (Z)- |
13.856 |
3.63 |
210.35984 |
C14H26O |
|
17 |
Trans-13-Octadecenoic acid |
14.050 |
1.63 |
282.46676 |
C18H34O2 |
|
18 |
Tetradecanal |
14.474 |
2.80 |
212.37572 |
C14H28O |
|
19 |
Oleic Acid |
14.674 |
1.08 |
282.46676 |
C18H34O2 |
|
20 |
15.612 |
3.00 |
282.46676 |
C18H34O2 |
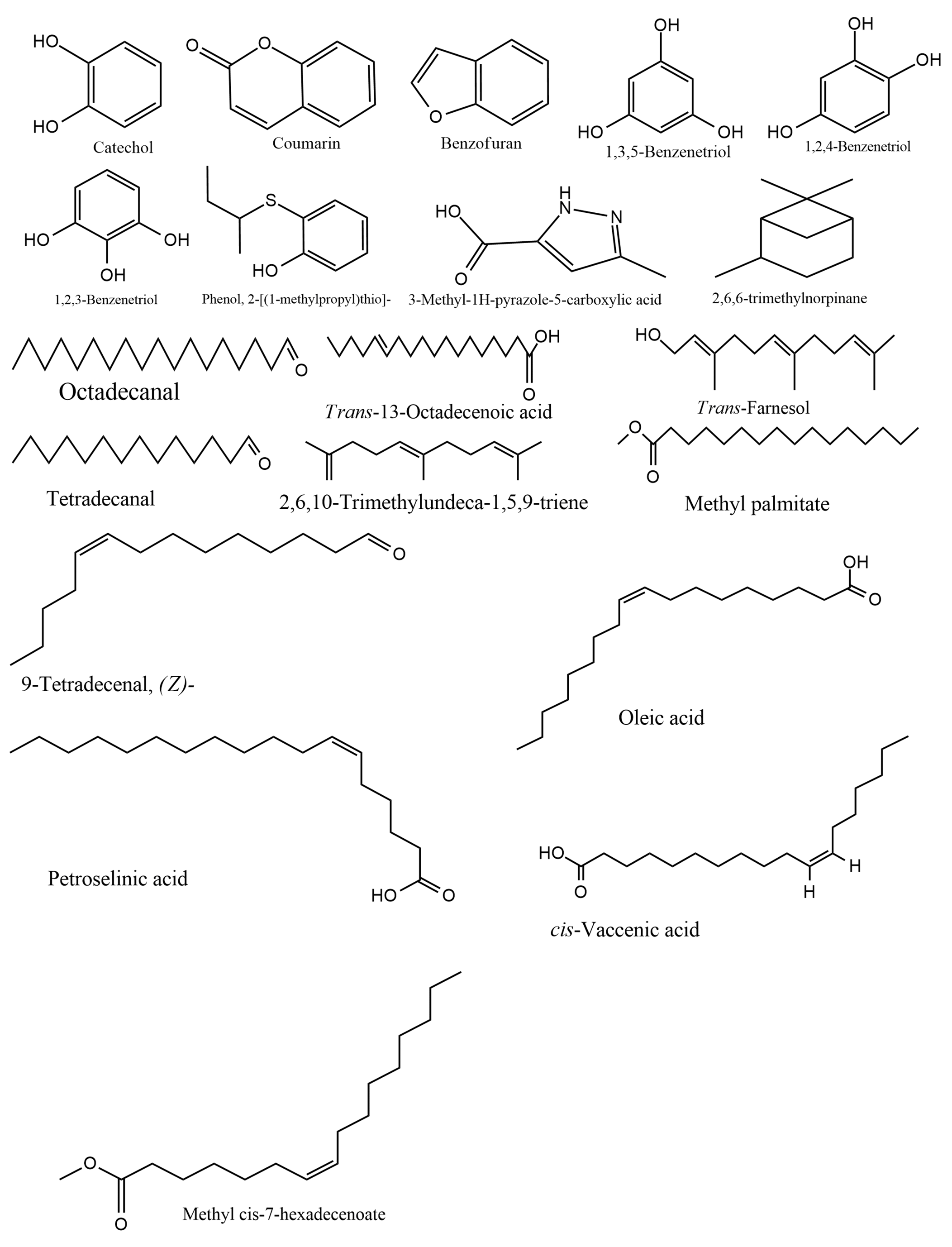
Figure 2. Structures of compounds identified in ESBE of D. microcarpum.
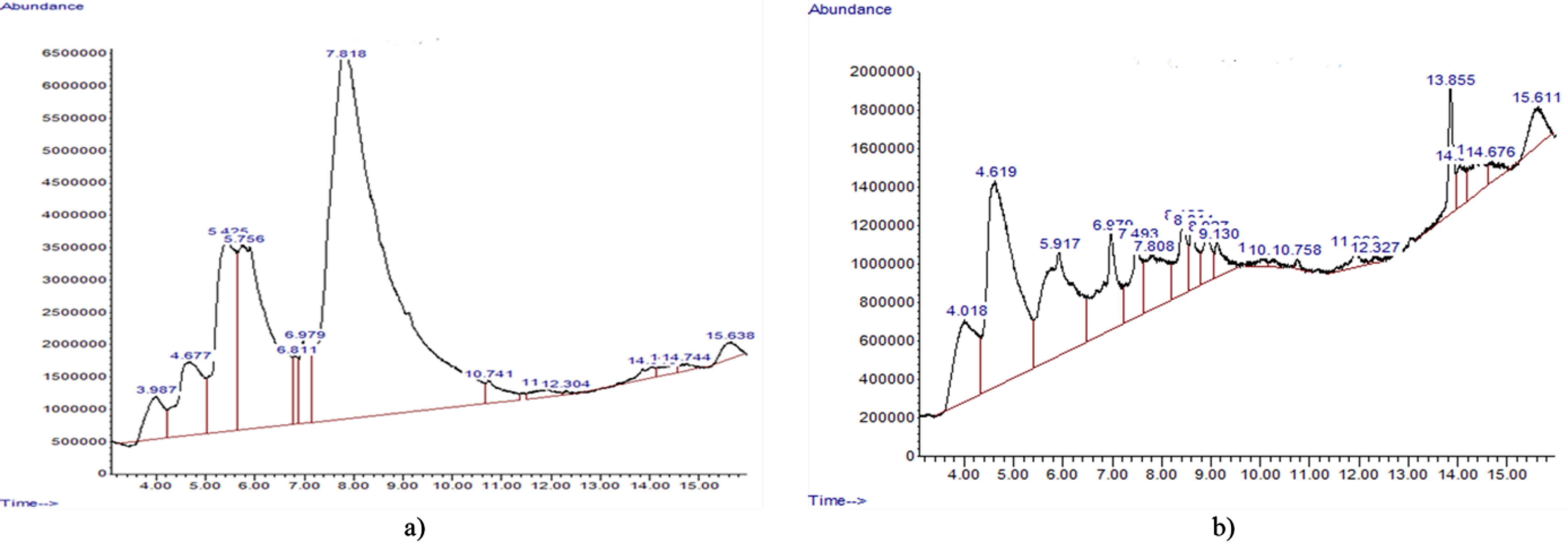
Figure 3. GS-MS Chromatogram of compounds identified in the; a) ESBE and b) ASBE of D. microcarpum.
Table 3 shows the compounds identified in the ESBE of D. microcarpum. The GCMS analysis identified 20 compounds in ESBE with coumarin being the most abundant (29.41%), followed by benzofuran (17.23%) and catechol (9.23%). The structures of the various compounds identified in the ESBE are presented in Figure 2, showing the different groups in the structures while Figure 3 shows the chromatogram of the ESBE, displaying the peak areas and retention times of the compounds identified.
Discussion
The phytochemicals present in the ASBE and ESBE of D. microcarpum are shown in Table 1. The absence of some phytochemicals in the aqueous extract though present in the ethanol extract might be due to the difference in solvent polarity as water is more polar than ethanol, thus less hydrophilic phytochemicals might be detected in the ethanol but not the aqueous extract (34). Phytochemicals found in plants have been attributed with different pharmacological roles which include antimicrobial, anti-ulcer, anti-diabetic, antioxidant, and anti-inflammatory activities through different mechanisms of action (35-37, 18, 38, 11, 39, 6, 33, 40). The alkaloid trigonelline extracted from Allium sepapea, Coffea sp, and Pissum sativum were reported to have several biological activities which include cardio-protective, hepato-protective, hypoglycemic, hypocholesterolemic, and anti-cancers activities (41). In another study, Ding et al., (42) reported alkaloids to be associated with antitumor effects and implicated in activities against microbes and inflammation. Mondal et al., (43) linked alkaloids to cancer phytotherapy and might serve as a good source for the development of anti-cancer therapeutics. Kohnen-Johannsen et al., (44) reported scopolamine (hyoscine) which is another alkaloid as a therapy for nausea and vomiting conditions.
Zhao et al., (45) that reported saponins possessed anti-tumor activities through multiple signaling-related pathways, subsequently leading to inhibition of proliferation, promotion of apoptosis, and regulation of tumor microenvironment. Several studies have reported plants having a high amount of saponins in association with cardioprotective pharmacological activities (46-48). Saponins raised the activity of several antioxidant enzymes responsible for cardiac protection against reactive oxygen species (46, 49-52). A saponin (trillin) acted through the anti-lipase mechanism to improve lipid profile by improving lipid peroxidation and superoxide dismutase activity (50). Yang et al., (53) reported dioscin as an active agent against a tumor, microbes, inflammation, and oxidative stress.
A Glycoside (phenylethanoid) isolated from natural sources was implicated in the therapy of tumors, inflammation, and microbial infections (54, 55). Glycosides from plant sources also possess anticancer properties, mediated through multiple mechanisms (56). Flavonoids exhibit hepatoprotective and antifibrotic activities, antimicrobial, antidiabetic, and anti-inflammatory activities (57). Flavonoids have been shown to exert effects against Escherichia coli, Bacillus cereus, and Staphylococcus aureus (58). The anticancer activity of flavonoids against human hepatocarcinoma by inducing apoptosis through the mitochondria-dependent apoptotic pathway has been previously reported by Hu et al., (59). The flavonoid quercetin exhibits anticancer and antiviral application in the management of allergy, inflammation, cardiovascular diseases, and arthritis and potential against Alzheimer's disease which is credited to its ability to inhibit acetylcholinesterase (60). A similar report on the methanol extract of D. microcarpum where alkaloids, saponins, glycoside, and flavonoids were detected agreed with our study (61). In another study, Abdullahi et al., (20) reported alkaloids, saponins, glycosides, and flavonoids in the alcohol extract of D. microcarpum. The present study obtained a similar result. A similar study on ethanol extract detected alkaloids, saponins, glycosides, and flavonoids (62) which agrees with the present study.
GCMS analysis revealed 14 compounds in the ASBE of the plant of study (Table 2). The compounds identified were aromatic compounds containing hydroxyl groups and long-chain aliphatic fatty acids. Coumaric acid is linked with several biological activities, including anti-cancer, antiviral, and antimicrobial activities. It is also implicated in the management of oxidative stress, inflammation, and arthritis with analgesic properties (63). A previous study demonstrated the antioxidant capacity of coumaric acid (64). In another study, coumaric acid demonstrated anti-fungal activity by retarding the growth of Fusarium oxysporum and Fusarium verticillioides (65). Anti-bacterial activity of coumaric acid against Streptococcus pneumonia, Staphylococcus aureus, and Bacillus subtilis through increased membrane permeability and binding to DNA was previously reported (66). Coumaric acid was previously associated with pharmacological activities against hepatitis C through impairment of entry and translation of the virus (67). Anti-viral activity of coumaric acid against the influenza virus by increasing the survival time was reported previously (68). Coumaric acid prevents mutagenesis caused by peroxide radicals which cause aberrations in chromosomes by scavenging the free radicals (69). The antidiabetic of coumaric acid was reported to be through stimulation of insulin secretion, decreased intestinal absorption of carbohydrates, and increased beta-cell activities (70). In a recent study, coumaric acids showed hepato-protective properties induced by carbon tetrachloride or bile duct ligation and exhibited amoebostatic activity against Entamoeba histolytica (71).
1,2,3-Benzenetriol otherwise called pyrogallol identified in both aqueous and ethanol extracts is associated with antibacterial activities against Staphylococcus aureus (72). The anti-malarial potential of pyrogallol investigated revealed that the compound can be a candidate for use against malarial parasites due to its auto-oxidation in the presence of metallic ions (Cu2+, Fe3+, and Mn2+) to produce free radicals, thus inhibiting the growth of parasite, a characteristic of anti-malarial drugs (72-74). Anti-bacterial study of pyrogallol reported inhibition of two strains of Staphylococcus aureus (75). Coumarin identified was identified in both aqueous and ethanol extracts in the present study have been linked with various pharmacological activities against fungi, bacteria, cancer, and inflammation. It acts as an anticoagulant and antioxidant and also has hypoglycemic and neuroprotective properties (76). The coumarin glycerol possesses anticancer properties through induction of apoptosis (77). Another coumarin butyrate demonstrated anticancer activity by causing apoptosis and inhibiting cancer cells of the colon (78). In an in vitro study, glycycoumarin demonstrated anticancer activity against hepatic cancer cells via apoptosis induction and shrinking the tumors (79). Glycycoumarin has also been linked with pharmacological activities which include antimicrobial, hepatoprotective, anti-inflammatory, antispasmodic, neuroprotective, antioxidant, and antiviral activities (80).
In the ethanol extract, 20 compounds were identified (Table 3). Some of these compounds were reported to be associated with various pharmacological activities. Benzofurans exert antimicrobial activities against fungi and bacteria and act as an anti-tumor anti-breast cancer agent (81). Catechol isolated from Semecarpus anacardium seeds was reported to have antioxidant and hypoglycemic activities (82). Methyl palmitate was previously reported to exert some pharmacological activities against inflammation in rats (83). In another study, methyl palmitate demonstrated pharmacological activity by decreasing inflammation through the reduction of the expression of cytokines, promoting inflammation, and increasing the expression of anti-inflammatory cytokines (84). Methyl palmitate shows antioxidant activity by reducing markers of oxidative stress and elevating the activities of the innate antioxidants (84). Methyl palmitate has been previously linked with insecticidal activity (85). Results similar to our study on GCMS analysis of D. microcarpum were previously obtained (86, 87).
Conclusion
The results of our study show that different phytochemicals are present in D. microcarpum which were previously linked to various pharmacological and biological activities. The compounds identified might be good synthetic sources of novel drugs against different ailments. Thus, this study justified the claims for the extensive use of D. microcarpum in traditional ethnomedicine. Additionally, further studies to isolate and determine the pharmacological activities of the compounds reported in our study are warranted.
Abbreviations
GCMS = Gas Chromatography-Spectrophotometry; ASBE = Aqueous Stembark Extract; ESBE = Ethanol Stembark Extract.
Declarations
Acknowledgment
The authors are highly thankful to Tertiary Education Trust Fund (TETfund) Nigeria for funding the research through the Institution-based research (IBR) fund of Adamawa State Polytechnic Yola. The authors also acknowledge the Department of Science Laboratory Technology, Adamawa State Polytechnic, for Institutional support and assistance
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
The present research was funded by the Tertiary Education Trust Fund (TETfund) of Nigeria through the Institution-based research (IBR) fund of Adamawa State Polytechnic Yola.
Conflict of Interest
The authors declare no conflicting interest.
References
- Emre G, Dogan A, Haznedaroglu MZ, Senkardes I, Ulger M, Satiroglu A, et al. An ethnobotanical study of medicinal plants in Mersin (Turkey). Front Pharmacol. 2021;12:664500
- Beniaich G, Salim R, Ech-Chihbi E, El-Hajjaji F, Rais Z, Abdellaoui A, et al. Ethnobotanical survey about medicinal plants used in traditional treatment of insomnia, asthenia, and oral and gum infections in the region Fez-Meknes, Morocco. Environmental Science and Pollution Research. 2022;29(1):133-45
- Dahiru MM, Nadro MS. Anti-diabetic potential of Hyphaene thebaica fruit in streptozotocin-induced diabetic rats. J Expl and Molec Biol. 2022;23(1):29-36.
- Dahiru MM. Recent advances in the therapeutic potential phytochemicals in managing diabetes. Journal of Clinical and Basic Research. 2023;7(1):13-20.
- Dahiru MM, Musa N, Abaka AM, Abubakar MA. Potential Antidiabetic Compounds from Anogeissus leiocarpus: Molecular Docking, Molecular Dynamic Simulation, and ADMET Studies. Borneo J Pharm. 2023;6(3):249-77.
- Dahiru MM, Abaka AM, Ya'u I. Antibacterial Potential of Ximenia americana L. Olacaceae: Molecular Docking, Molecular Dynamics, and ADMET Prediction. Journal of Pharmacy. 2024;4(1):51-67.
- Dahiru MM, Alfa MB, Abubakar MA, Abdulllahi AP. Assessment of in silico antioxidant, anti-inflammatory, and antidiabetic activites of Ximenia americana L. Olacaceae. Advances in Medical, Pharmaceutical and Dental Research. 2024;4(1):1-13.
- Slimani I, Najem M, Belaidi R, Bachiri L, Bouiamrine EH, Nassiri L, et al. Étude ethnobotanique des plantes médicinales utilisées dans la région de Zerhoun-Maroc-[Ethnobotanical Survey of medicinal plants used in Zerhoun region-Morocco-]. International Journal of Innovation and Applied Studies. 2016;15(4):846.
- Belyagoubi-Benhammou N, Belyagoubi L, Bechlaghem N, Ghembaza N, Atik-Bekkara F. Assessment of antioxidant potential and phytochemical analysis of Pituranthos scoparius crude extract and its fractions. Orient Pharm Exp Med. 2017;17(1):51-7
- Dahiru MM, Nadro SM. Hypolipidemic Potential of Ethyl acetate Extract of Hyphaene thebaica Fruit in Streptozotocin-induced Diabetic Rats. Majalah Obat Tradisional. 2022;27(2):159-64.
- Dahiru MM, Ahmadi H, Faruk MU, Aminu H, Hamman, Abreme GC. Phytochemical Analysis and Antioxidant Potential of Ethylacetate Extract of Tamarindus Indica (Tamarind) Leaves by Frap Assay. Journal of Fundamental and Applied Pharmaceutical Science. 2023;3(2):45-53.
- Musa N, Dahiru MM, Badgal EB. Characterization, In Silico Antimalarial, Antiinflammatory, Antioxidant, and ADMET Assessment of Neonauclea excelsa Merr. Sciences of Pharmacy. 2024;3(2):92-107.
- Dayamba SD, Savadogo P, Sawadogo L, Zida D, Tiveau D, Oden PC. Dominant species’ resprout biomass dynamics after cutting in the Sudanian savanna-woodlands of West Africa: long term effects of annual early fire and grazing. Annals of Forest Science. 2011;68(3):555-64
- Meda NR, Fraisse D, Gnoula C, Vivier M, Felgines C, Senejoux F. Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. European Food Research and Technology. 2017;243(9):1659-66
- Habibou HH, Abdoulahi MII, Moctar C, Zakari CO, Rahila HG, Khalid I. Phytochemistry and pharmacology activities of Detarium microcarpum (Fabaceae) used in the treatment of parasitic diseases in Niger: A review. Journal of Pharmacognosy and Phytochemistry. 2022;11(2):60-7.
- Kurmi U, Modu B, Patel P, Uthman R, Balkore H, Umar R, et al. Phytochemical Screening and In-Vitro Antioxidant Activity of Ethanolic Leaves Extract of Detarium microcarpum. World Journal of Pharmaceutical Research. 2021;10:84.
- Sanusi SB, Lawal SM, Usman A, Musa FM, Ardo B, Way TB. Phytochemical Analysis and Antibacterial Activity of Stem Bark Extracts of Detarium Microcarpum Against Bacteria Causing Gastrointestinal Tract Infections in Humans. Dutse Journal of Pure and Applied Sciences. 2022;8(1b):82-9.
- Dahiru MM, Abaka AM, Artimas SP. Phytochemical Analysis and Antibacterial Activity of Methanol and Ethyl Acetate Extracts of Detarium microcarpum Guill. & Perr. Biology, Medicine, & Natural Product Chemistry. 2023;12(1):281-8.
- Mbock MA, Fouatio WF, Kamkumo RG, Tsouh Fokou PV, Tsofack FN, Lunga P-K, et al. In vitro and in vivo anti-salmonella properties of hydroethanolic extract of Detarium microcarpum Guill. & Perr. (Leguminosae) root bark and LC-MS-based phytochemical analysis. J Ethnopharmacol. 2020;260:113049.
- Abdullahi AR, Malami S, Alhassan Bichi L. In Vivo Antiplasmodial Activity of Detarium microcarpum (Fabaceae) Stem Bark Extract %G en. 2021;10(1 %9 Research Article):e116921.
- Akah PA, Nworu CS, Mbaoji FN, Nwabunike IA, Onyeto CA. Genus Detarium: Ethnomedicinal, phytochemical and pharmacological profile. Phytopharmacology. 2012;3(2):367-75.
- Salawu KM, Ogbole OO, Abiodun OO, Ajaiyeoba EO. Ethnobotanical Survey, Phytochemical Screening, Growth Inhibitory Effects and Cytotoxicity Evaluation of Medicinal Plants used for Cancer Management in Ilorin Metropolis, Nigeria. Archives of Basic and Applied Medicine. 2021;9(2):168–75.
- Manukumar HM, Shiva Kumar J, Chandrasekhar B, Raghava S, Umesha S. Evidences for diabetes and insulin mimetic activity of medicinal plants: present status and future prospects. Crit Rev Food Sci Nutr. 2017;57(12):2712-29
- Saidu IN, Umar KS, Isa MH. Ethnobotanical survey of anticancer plants in Askira/Uba local government area of Borno State, Nigeria. African Journal of Pharmacy and Pharmacology. 2015;9(5):123-30
- Dahiru MM, Abdulhasib Oluwatobi O, James D, Aisha Alfa A, Faith J, Alkasim Yahaya H, et al. An In Vitro Assessment of the Antioxidant Activity of Detarium microcarpum Guill. & Perr. Fabaceae. Sciences of Phytochemistry 2024;3(2):114-22.
- Evans WC. Trease and Evans' pharmacognosy: Elsevier Health Sciences; 2009. 608 p.
- Harborne A. Phytochemical methods a guide to modern techniques of plant analysis: springer science & business media; 1998.
- Obadoni B, Ochuko P. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Global J Pure Appl Sci. 2002;8(2):203-8.
- Ugwoke C, Orji J, Anze S, Ilodibia C. Quantitative phytochemical analysis and antimicrobial potential of the ethanol and aqueous extracts of the leaf, stem and root of Chromolaena odorata (Asteraceae). International Journal of Pharmacognosy and Phytochemical Research. 2017;9(2):207-14.
- Dahiru MM, Badgal EB, Musa N. Phytochemistry, GS-MS analysis, and heavy metals composition of aqueous and ethanol stem bark extracts of Ximenia americana. GSC Biol Pharm Sci. 2022;21(3):145-56.
- Badgal EB, Dahiru MM, Musa N. Phytochemical profiling, heavy metals composition, in silico aphrodisiac potential, and ADMET study of Gardenia erubescens. Sciences of Phytochemistry. 2023;2(2):91-106.
- Dahiru MM, Badgal EB, Neksumi M. Phytochemical profiling and heavy metals composition of aqueous and ethanol extracts of Anogeissus leiocarpus. Journal of Faculty of Pharmacy of Ankara University. 2023;47(2):311-23.
- Dahiru MM, Musa N. Phytochemical Profiling, Antioxidant, Antidiabetic, and ADMET Study of Diospyros mespiliformis Leaf, Hochst Ex A. Dc Ebenaceae. J Fac Pharm Ankara/Ankara Ecz Fak Derg. 2024;48(2):412-35.
- Aboshora W, Lianfu Z, Dahir M, Qingran M, Qingrui S, Jing L, et al. Effect of extraction method and solvent power on polyphenol and flavonoid levels in Hyphaene thebaica L mart (Arecaceae)(Doum) fruit, and its antioxidant and antibacterial activities. Tropical Journal of Pharmaceutical Research. 2014;13(12):2057-63 %@ 1596-9827.
- Doughari JH. Phytochemicals: extraction methods, basic structures and mode of action as potential chemotherapeutic agents: INTECH Open Access Publisher Rijeka, Croatia; 2012.
- Alhassan DA, Uba AI, Muhammad AU, Muhammad YYu. Phytochemical screening and antimicrobial activity of crude stem bark extracts of Anogeissus leiocarpus. European Journal of Medicinal Plants. 2016;11(2):1-7.
- Dahiru MM, Nadro MS. Phytochemical Composition and Antioxidant Potential of Hyphaene thebaica Fruit. Borneo J Pharm. 2022;5(4):325-33.
- Dahiru MM, Abaka AM, Musa N. Phytochemical Analysis, In-vitro, and In-silico Antibacterial Activity of Stembark Extract of Anogeissus leiocarpus (DC) Guill and Perr. Sciences of Pharmacy. 2023;2(3):24-41.
- Abaka AM, Dahiru MM, Abubakar KB, Luka J, Abubakar A, Abdullahi TB, et al. Phytochemical Profile and Antibacterial Activity of Nigella Sativa against Biofilm-producing Bacteria Uropathogens. Biology, Medicine, & Natural Product Chemistry. 2024;13(1):141-6.
- Dahiru MM, Umar AS, Muhammad M, Waziri AuA, Fari II, Musa ZY. Phytoconstituents, Fourier-Transform Infrared Characterization, and Antioxidant Potential of Ethyl Acetate Extract of Corchorus olitorius (Malvaceae). 2024;3(1):1-10.
- Mohamadi N, Sharififar F, Pournamdari M, Ansari M. A Review on Biosynthesis, Analytical Techniques, and Pharmacological Activities of Trigonelline as a Plant Alkaloid. J Diet Suppl. 2018;15(2):207-22.
- Ding Y, Qu D, Zhang K-M, Cang X-X, Kou Z-N, Xiao W, et al. Phytochemical and biological investigations of Amaryllidaceae alkaloids: a review. J Asian Nat Prod Res. 2017;19(1):53-100.
- Mondal A, Gandhi A, Fimognari C, Atanasov AG, Bishayee A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur J Pharmacol. 2019;858:172472.
- Kohnen-Johannsen KL, Kayser O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production Molecules. 2019;24(4):796.
- Zhao Y-Z, Zhang Y-Y, Han H, Fan R-P, Hu Y, Zhong L, et al. Advances in the antitumor activities and mechanisms of action of steroidal saponins. Chinese Journal of Natural Medicines. 2018;16(10):732-48.
- Haleagrahara N, Varkkey J, Chakravarthi S. Cardioprotective effects of glycyrrhizic acid against isoproterenol-induced myocardial ischemia in rats. Int J Mol Sci. 2011;12(10):7100-13
- Zhang R, Fang W, Han D, Sha L, Wei J, Liu L, et al. Clematichinenoside attenuates myocardial infarction in ischemia/reperfusion injury both in vivo and in vitro. Planta Med. 2013;79(14):1289-97
- Li S, Zhao J, Liu Y, Chen Z, Xu Q, Khan IA, et al. New triterpenoid saponins from Ilex cornuta and their protective effects against H2O2-induced myocardial cell injury. Journal of Agricultural and Food Chemistry. 2014;62(2):488-96
- Li C, Gao Y, Tian J, Xing Y, Zhu H, Shen J. Long-term oral Asperosaponin VI attenuates cardiac dysfunction, myocardial fibrosis in a rat model of chronic myocardial infarction. Food Chem Toxicol. 2012;50(5):1432-8
- Wang T, Choi RCY, Li J, Bi CWC, Ran W, Chen X, et al. Trillin, a steroidal saponin isolated from the rhizomes of Dioscorea nipponica, exerts protective effects against hyperlipidemia and oxidative stress. J Ethnopharmacol. 2012;139(1):214-20
- Wu J, Yang G, Zhu W, Wen W, Zhang F, Yuan J, et al. Anti-atherosclerotic activity of platycodin D derived from roots of Platycodon grandiflorum in human endothelial cells. Biological and Pharmaceutical Bulletin. 2012;35(8):1216-21
- Wang M, Meng X-b, Yu Y-l, Sun G-b, Xu X-d, Zhang X-p, et al. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis. 2014;19(12):1727-35
- Yang L, Ren S, Xu F, Ma Z, Liu X, Wang L. Recent Advances in the Pharmacological Activities of Dioscin. Biomed Res Int. 2019;2019:5763602.
- Xue Z, Yang B. Phenylethanoid Glycosides: Research Advances in Their Phytochemistry, Pharmacological Activity and Pharmacokinetics. Molecules [Internet]. 2016; 21(8).
- Tian X-Y, Li M-X, Lin T, Qiu Y, Zhu Y-T, Li X-L, et al. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur J Med Chem. 2021;209:112563.
- Khan H, Saeedi M, Nabavi SM, Mubarak MS, Bishayee A. Glycosides from medicinal plants as potential anticancer agents: Emerging trends towards future drugs. Curr Med Chem. 2019;26(13):2389-406 %@ 0929-8673.
- Bratkov VM, Shkondrov AM, Zdraveva PK, Krasteva IN. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. 2016(0973-7847 (Print)).
- Chen J, Li Y, Yang L-Q, Li Y-Z, Nan Z-B, Gao K. Biological activities of flavonoids from pathogenic-infected Astragalus adsurgens. Food Chem. 2012;131(2):546-51
- Hu Y-W, Liu C-Y, Du C-M, Zhang J, Wu W-Q, Gu Z-L. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J Ethnopharmacol. 2009;123(2):293-301.
- Batiha GE-S, Beshbishy AM, Ikram M, Mulla ZS, El-Hack MEA, Taha AE, et al. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin Foods 2020;9(3):374.
- David J, Afolabi EO, Olotu PN, Ojerinde SO, Agwom FM, Ajima U. Phytochemical analysis, antidiabetic and toxicity studies of the methanolic leaf extract of Detarium microcarpum guill and perr in wistar albino rats. J Chem Pharm Res. 2017;9(11):55-60.
- Kurmia UG, Modua B, Patelb P, Abubakar F, Umara RB, Balkorea HA, et al. Phytochemical Screening and In-Vitro Antioxidant Activity of Ethanolic Leaves Extract of Detarium microcarpum. World Journal of Pharmaceutical Research 2021;10(5):1698-710.
- Pei K, Ou J, Huang J, Ou S. p‐Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. Journal of the Science of Food and Agriculture. 2016;96(9):2952-62
- Mathew S, Abraham TE, Zakaria ZA. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol. 2015;52(9):5790-8
- Zabka M, Pavela R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere. 2013;93(6):1051-6
- Lou Z, Wang H, Rao S, Sun J, Ma C, Li J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012;25(2):550-4
- Tanida I, Shirasago Y, Suzuki R, Abe R, Wakita T, Hanada K, et al. Inhibitory effects of caffeic acid, a coffee-related organic acid, on the propagation of hepatitis C virus. Jpn J Infect Dis. 2015;68(4):268-75
- Kai H, Obuchi M, Yoshida H, Watanabe W, Tsutsumi S, Park YK, et al. In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08). J Funct Foods. 2014;8:214-23
- Ferguson LR, Lim IF, Pearson AE, Ralph J, Harris PJ. Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2003;542(1-2):49-58
- Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. Journal of diabetes & metabolic disorders. 2013;12(1):1-9
- Aldaba‑Muruato LR, Ventura‑Juárez J, Perez‑Hernandez AM, Hernández‑Morales A, Muñoz‑Ortega MH, Martínez‑Hernández SL, et al. Therapeutic perspectives of <em>p</em>‑coumaric acid: Anti‑necrotic, anti‑cholestatic and anti‑amoebic activities. World Acad Sci J. 2021;3(5):47.
- Cynthia FI, Hery S, Akhmad D. Antibacterial and antioxidant activities of pyrogallol and synthetic pyrogallol dimer. Res J Chem Environ. 2018;22:39-47.
- Upadhyay G, Gupta SP, Prakash O, Singh MP. Pyrogallol-mediated toxicity and natural antioxidants: triumphs and pitfalls of preclinical findings and their translational limitations. Chem Biol Interact. 2010;183(3):333-40
- Percário S, Moreira DR, Gomes BAQ, Ferreira MES, Gonçalves ACM, Laurindo PSOC, et al. Oxidative stress in malaria. Int J Mol Sci. 2012;13(12):16346-72.
- Oliveira LCC, Rodrigues FAA, dos Santos Barbosa CR, dos Santos JFS, Macêdo NS, de Sousa Silveira Z, et al. Antibacterial Activity of the Pyrogallol against Staphylococcus aureus Evaluated by Optical Image. Biologics. 2022;2(2):139-50
- Venugopala KN, Rashmi V, Odhav B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. Biomed Res Int. 2013;2013.
- Zang Y. Pharmacological activities of coumarin compounds in licorice: a review. Nat Prod Commun. 2020;15(9):1934578X20953954
- Pant K, Yadav AK, Gupta P, Islam R, Saraya A, Venugopal SK. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biology. 2017;12:340-9
- Song X, Yin S, Zhang E, Fan L, Ye M, Zhang Y, et al. Glycycoumarin exerts anti-liver cancer activity by directly targeting T-LAK cell-originated protein kinase. Oncotarget. 2016;7(40):65732.
- Zang Y. Pharmacological Activities of Coumarin Compounds in Licorice: A Review. Natural Product Communications. 2020;15(9):1934578X20953954.
- Khodarahmi G, Asadi P, Hassanzadeh F, Khodarahmi E. Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents: A review. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2015;20 (11):1735-995.
- Sundaram RA-O, Muthu K, Shanthi P, Sachdanandam P. Antioxidant and antihyperlipidemic activities of catechol derivatives and biflavonoid isolated from Semecarpus anacardium seeds. Toxicology mechanisms and methods. 2022;32(2):123-31.
- Saeed NM, El-Demerdash E, Abdel-Rahman HM, Algandaby MM, Al-Abbasi FA, Abdel-Naim AB. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicology and applied pharmacology. 2012;264(1):84-93
- Hamed A, Mantawy E, El-Bakly W, Abdel-Mottaleb Y, Azab S. Methyl Palmitate: the Naturally Occurring Cardioprotective Agent. Archives of Pharmaceutical Sciences Ain Shams University. 2020;4(1):47-62
- Wang YN, Wang HX, Jin YS, Bu CY, Cheng J, Zhao LL, et al. Assessment of the contact toxicity of methyl palmitate on Tetranychus viennensis (Acari: Tetranychidae). J Econ Entomol. 2010;103(4):1372-7
- Stanley I, Ikpa C, Njoku VO, Kalu G, Isiuku B, Nwangwu S. GC-MS Analysis of Phytochemical Composition, Antibacterial and Antioxidant Properties of Ethanolic Extract of Detarium Microcarpum (OFOR) GC-MS Analysis of Phytochemical Composition, Antibacterial and Antioxidant Properties of Ethanolic Extract of Detarium microcarpum (OFOR). Medicinal and Analytical Chemistry International Journal. 2020;4(1):000158.
- Dahiru M, Saheed KO, Adamu TM. Antibacterial and phytochemicals status of Detarium microcarpum (guill and perr) stem bark. Natural Resources for Human Health. 2022;2(4):385-90.

 ETFLIN
Notification
ETFLIN
Notification






