Formulation and Characterization of Resveratrol-Loaded Nanostructured Lipid Carriers (NLC) with Mesua ferrea Seed Oil as Liquid Lipid
by Madhuchandra Lahan ★
Academic editor: Farid Menaa
Sciences of Pharmacy 3(4): 203-211 (2024); https://doi.org/10.58920/sciphar0304271
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
12 Aug 2024
13 Nov 2024
01 Dec 2024
11 Dec 2024
Abstract: Nanostructured Lipid Carriers (NLCs) are colloidal drug delivery systems composed of both solid and liquid lipids. They enhance drug loading capacity, regulate the release of poorly water-soluble drugs, and are suitable for targeted delivery. Resveratrol, a polyphenol with promising anticancer properties, faces challenges due to its low water solubility, poor bioavailability, and chemical instability, resulting in rapid metabolism and excretion. Therefore, it is crucial to develop a delivery system that safeguards resveratrol during its transit through the body. This study aimed to develop and characterize resveratrol-loaded NLCs using the nano-precipitation method followed by ultrasonication, incorporating Mesua ferrea seed oil as the liquid lipid. The NLCs were evaluated for particle size, morphology (TEM), zeta potential, drug entrapment efficiency, drug loading, and in vitro drug release. The resulting NLCs demonstrated stability and homogeneity, with a particle size of 181.6 ± 12.4 nm, a polydispersity index (PDI) of 0.135 ± 0.09, drug entrapment efficiency of 82.76 ± 12.2%, and drug loading capacity of 42.94 ± 7.5%. They exhibited sustained drug release, achieving 84.56% release within 24 h. These findings suggest that the developed NLCs can effectively enhance the incorporation and controlled release of poorly water-soluble drugs like resveratrol, offering potential advantages over conventional delivery systems.
Keywords: Nanostructured Lipid CarrierResveratrolMesua ferreaNanoparticles
Introduction
In recent years, the development of lipid-based drug carriers has garnered significant attention due to their promising potential in drug delivery systems (1). Nanoparticles, colloidal particles ranging in size from 10 to 1000 nm, are particularly noteworthy for their ability to enhance therapeutic efficacy through size-dependent properties (2). Lipid nanoparticles offer an innovative approach to drug delivery by enabling secondary and tertiary targeting. Their unique structure facilitates targeted and controlled drug release, making them highly desirable for advanced therapeutic applications (1). Lipid nanoparticles possess several advantageous features: (i) the ability to encapsulate both lipophilic and hydrophilic drugs, (ii) low toxicity, (iii) controlled drug release capabilities, (iv) biodegradability, producing non-toxic degradation products, (v) protection of encapsulated drugs from degradation, (vi) scalability and ease of manufacturing, and (vii) the low cost of lipids and stabilizers. These characteristics make them an attractive option for drug delivery (2, 3).
Nanostructured Lipid Carriers (NLCs) are a subclass of lipid nanoparticles composed of a binary mixture of solid and liquid lipids, typically in ratios ranging from 70:30 to 99.9:0.1 (4-6). The inclusion of liquid lipids enhances drug loading and stability. NLCs surpass traditional lipid formulations by improving drug encapsulation, minimizing leakage, regulating release, and reducing adverse effects of organic solvents (7, 8). The formulation process integrates the liquid lipid within the solid lipid matrix, thereby increasing drug-loading capacity, reducing drug expulsion during storage, and enabling controlled release. NLCs can be formulated for various administration routes, including oral, intravenous, pulmonary, and transdermal, making them versatile carriers for targeted drug delivery and protection of bioactive compounds from degradation (5, 9, 10). Furthermore, NLCs can be prepared for multiple routes of administration, including oral, intravenous, pulmonary, and transdermal formulations. NLCs are suitable for transporting the drug to the specific target sites and protecting important bioactive compounds against degradation. These are considered safe and ideal for incorporating lipophilic and poorly water-soluble compounds such as Resveratrol (9, 11).
Mesua ferrea L. (family Clusiaceae) is a rich source of secondary metabolites with numerous pharmacological benefits. Traditionally, it has been used as an astringent, stomachic, expectorant, antioxidant, analgesic, anti-inflammatory, antitumor, and immunostimulant, among other applications. The seed oil of M. ferrea exhibits antioxidant, antimicrobial, immunomodulatory, antiarthritis, and anti-inflammatory activities (12-14). Beyond medicinal uses, the oil is employed as a biofuel, biolubricant, excipient in pharmaceuticals, and even as a substitute for gasoline. Given the rising demand for edible oils such as peanut, corn, and sesame due to population growth, identifying alternative natural oils like M. ferrea seed oil is essential to reduce dependence on edible oils and lower pharmaceutical formulation costs (15). This study leverages M. ferrea seed oil as a liquid lipid carrier for hydrophobic drugs in NLC formulations
Resveratrol (3,5,4′-trihydroxystilbene) is a natural polyphenol found in grape skins and seeds, with extensive pharmacological benefits, including chemopreventive, cardiovascular, neuroprotective, antiviral, anti-inflammatory, antioxidant, and antitumor effects. It also shows potential in preventing diabetes and obesity (16-18). Resveratrol exerts its anticancer effects by scavenging free radicals, protecting DNA, and inducing apoptosis through polyamine metabolism and cell cycle regulation (17, 19). However, its therapeutic potential is limited by poor bioavailability, low water solubility, chemical instability, and rapid metabolism and excretion. Therefore, developing a robust delivery system to protect resveratrol during transit within the body is critical (19). The most important is developing a drug delivery system that protects resveratrol during its transit inside the organism (20). In this study, a novel resveratrol-loaded NLC was developed using M. ferrea seed oil as the liquid lipid for encapsulation. This approach aims to establish a safe, effective, and cost-efficient alternative excipient for pharmaceutical applications, enhancing resveratrol's stability, bioavailability, and therapeutic efficacy.
Materials and
Methods
Materials
Resveratrol was purchased from Oakwood Products (West Columbia, USA), Precirol ATO 5 and Compritol 888 ATO were purchased from Gattefosse (Germany). We also used stearic acid (Loba Chemie Pvt. Ltd, Mumbai), polyethylene glycol 400 obtained from Rankem (New Delhi, India). Methanol, acetone, pluronic F-68, dialysis membrane 70, and diethyl ether were obtained from Hi Media Laboratories (Mumbai, India). All the reagents and chemicals used were of analytical grades (AR) and complied with pharmacopoeial standards.
Collection and Extraction of Mesua ferrea Seed Oil
About 10 kg of the ripe fruits of M. ferrea were collected from Dibrugarh district of Assam in August 2023. The fruits were carefully plucked from trees, seeds were separated from the fruits, deshelled manually, and washed several times with water to remove the adhesive materials. Then, the seeds were dried in the sunlight for four days. The sun-dried seeds were finally crushed into fine powder and dried in an oven at 105°C for an hour. The powders were packed in clean, air-tight plastic containers (15).
The oil was extracted using a solvent extraction method using a soxhlet apparatus (15). The oil was extracted with light petroleum ether (40-60°C) from about 80 g of powdered seed material for 24 h. The solvent was recovered by distillation method, and the remaining solvent was removed in a water bath at 60°C for 1 h. After the oil was gathered, the percentage of oil content was determined. Percentage yield was calculated by using the formula, % Yield = weight of extract / weight of powdered seed x 100%.
Physicochemical Analysis of Mesua ferrea Seed Oil
Specific Gravity
A density bottle was utilized to find the oil's density. A clean and dry bottle of 25 mL capacity was weighed (W0) and then filled with the oil. Stopper was inserted and reweighed (W1). The oil was substituted with water after washing and drying the bottle and weighed (W2) (21). The specific gravity was calculated using Equation 1.
Equation 1
Ash Value (Total Ash)
Two grams of the air-dried extract were accurately weighed in a tared platinum or silica dish and incinerated at 450–500°C. After incineration, the carbon-free ash was weighed, and the percentage of ash was calculated (22).
Moisture Content
Two grams of the crude powder (Wi) were initially weighed separately and placed in an oven at 80°C for 6 h. The weight was measured every hour. This process was repeated until a constant weight was achieved (Wd). After each hour, the samples were removed from the oven and placed in desiccators for 30 min to cool. They were then reweighed. The moisture content (%MC) in the powder was calculated using Equation 2 (21).
Equation 2
Acid
Value
Approximately 10 g of M. ferrea seed oil was dissolved in 50 mL of a mixture of equal parts ethanol (95%) and ether, pre-neutralized with 0.1 M potassium hydroxide using phenolphthalein as an indicator. One milliliter of phenolphthalein solution was added, and the mixture was titrated with 0.1 M potassium hydroxide until it remained faintly pink after shaking for 30 s (22). The acid value was calculated using Equation 3.
Equation 3
Where, n = volume of 0.1 M potassium hydroxide in mL and w = weight of the substance (g).
Saponification
Value
About 2 g of the oil (w) was accurately weighed and placed into a 200-mL borosilicate glass flask fitted with a reflux condenser. Then, 25.0 mL of 0.5 M ethanolic potassium hydroxide was added, and the mixture was boiled under reflux in a water bath for 30 min. One milliliter of phenolphthalein solution was added, and the solution was immediately titrated with 0.5 M hydrochloric acid (a). A blank determination was performed, omitting the substance under examination (b) (22). The value of saponification (SV) was determined using Equation 4.
Equation 4
Ester
Value
The ester value was calculated using Equation 5.
Equation 5
Unsaponification Value
Two grams of the oil sample, along with 20 mL of alcoholic potassium hydroxide solution, were refluxed for 1 h or until saponification was complete. The saponified mixture was transferred to a separating funnel and washed with ethyl alcohol, followed by cold water. The temperature of the mixture was maintained at approximately 20 to 25ºC. Fifty milliliters of petroleum ether were added with vigorous shaking, and the layers were allowed to separate. After transferring the lower soap layer into the separating funnel, three additional extractions were performed using 50 mL volumes of petroleum ether. The residue was neutralized in 50 mL of warm ethanol, and the procedure was repeated for the saponification value. The solution was then titrated with 0.02N NaOH. The unsaponification value (UV) was calculated using Equation 6.
Equation 6
Where, A = weight of the residue in gram, B = weight of free fatty acid of extract in gram, and W = weight of the sample in gram.
Peroxide Value
After accurately weighing 5 g of the oil (w), it was placed in a 250 mL glass-stoppered conical flask. Thirty milliliters of a mixture of three parts glacial acetic acid and two parts chloroform were added, and the flask was swirled until the oil dissolved. Then, 0.5 mL of saturated potassium iodide solution was added. The mixture was allowed to stand for exactly 1 min, with occasional shaking. Next, 30 mL of water was added, and the solution was titrated gradually with 0.01 M sodium thiosulphate, shaking continuously and vigorously until the yellow color almost disappeared.
Afterward, 0.5 mL of starch solution was added, and titration continued with vigorous shaking until the blue color just disappeared (a in mL). A blank determination was performed, omitting the substance under examination (b in mL). The 0.01 M sodium thiosulphate volume used in the blank determination should not exceed 0.1 mL (22). The peroxide value was calculated using Equation 7.
Equation 7
Iodine Value
In an iodine flask, 0.2 g of oil was added, followed by 10 mL of carbon tetrachloride, and the mixture was dissolved. Then, 20 mL of iodine monochloride solution was added, the stopper was inserted, and the flask was allowed to stand in the dark between 15°C and 25°C for 30 min.
Next, 15 mL of potassium iodide solution was placed in the cup, the stopper was carefully removed, and the stopper and the sides of the flask were rinsed with 100 mL of water. The mixture was shaken and titrated with 0.1 M sodium thiosulphate, using starch solution as an indicator. The volume of sodium thiosulphate required was recorded as (a). The procedure was repeated without the substance under examination, and the volume of sodium thiosulphate required was recorded as (b) (22). Expression for iodine value was calculated using Equation 8.
Equation 8
Preparation of Resveratrol-Loaded NLC
The resveratrol-loaded NLC was prepared using the nano-precipitation method, followed by the ultrasonication method, with different parameters as outlined in Table 1. The nanoprecipitation technique is based on interfacial deposition due to the displacement of a solvent with a non-solvent. It is a simple, less complex, energy-efficient, and widely applicable technique (23). Briefly, resveratrol (10 mg/mL) was dissolved in 2 mL of a suitable organic solvent containing a mixture of solid and liquid lipids (40 mg, 70:30). The solution was magnetically stirred at 300 rpm. The organic solution was then rapidly injected through an injection needle into 40 mL of distilled water containing the surfactant Pluronic F-68 (1% w/v), stirring continuously at 1500 rpm for 15 min (24). The solution was then ultrasonicated for 20 min using an ultrasonic cleaning bath (Model UCB 30, 20 Hz power). The organic solvent was allowed to evaporate at room temperature under continued magnetic stirring for 24 h. The nanoparticles were then collected, and characterization was performed.
Table 1. Composition of various NLC formulations.
|
Parameters |
Code |
Solvents |
Liquid lipid |
Solid lipid |
Organic: aqueous phase ratio |
Ultrasonication time (min)
|
|
Solvent system
(Organic phase) |
F1 |
Acetone
|
M. ferrea oil |
Precirol ATO 5 |
1:20 |
20 |
|
F2 |
Acetone + isopropanol (8:2)
|
M. ferrea oil |
Precirol ATO 5 |
1:20 |
20 |
|
|
F3 |
Acetone + ethanol (8:2)
|
M. ferrea oil |
Precirol ATO 5 |
1:20 |
20 |
|
|
F4 |
Cyclohexane + Acetone (1:1)
|
M. ferrea oil |
Precirol ATO 5
|
1:20 |
20 |
|
|
Ratios of organic: aqueous phase |
F5 |
Acetone + isopropanol (8:2) |
M. ferrea oil |
Precirol ATO 5 |
1:10 |
20 |
|
F6 |
Acetone + isopropanol (8:2) |
M. ferrea oil
|
Precirol ATO 5 |
1:30 |
20 |
|
|
Different solid lipids |
F7 |
Acetone + isopropanol (8:2) |
M. ferrea oil |
Stearic acid
|
1:20 |
20 |
|
F8 |
Acetone + isopropanol (8:2) |
M. ferrea oil |
Precerol |
1:20 |
20 |
|
|
Increasing ultrasonicati-on time |
F9 |
Acetone + isopropanol (8:2) |
M. ferrea oil |
Precerol |
1:20 |
30 |
Characterization
of NLC
The prepared NLCs were characterized by determining particle size, polydispersity index (PDI), zeta potential, drug entrapment efficiency, and drug loading.
Particle Size, Size Distribution, and Zeta Potential
Particle size and polydispersity index were determined by dynamic light scattering (DLS), also known as photon correlation spectroscopy, using a particle size analyzer (Brookhaven Instruments, 90 plus, USA). The zeta potential was determined by measurement of the electrophoretic mobility using a zeta sizer (Malvern zetasizer nano ZS 90) at 25°C.The samples were diluted 100 times using distilled water before analysis, and the measurement was made 3 times, using data acquisition for 3 min at room temperature (25, 26).
Drug Entrapment Efficiency and Drug Loading
To determine the drug entrapment efficiency and drug loading, 10 mL of the prepared drug-loaded suspension was centrifuged at 8000 rpm for 30 min to separate the NLCs from the aqueous phase. One milliliter of the clear supernatant was diluted to 10 mL with methanol and analyzed spectrophotometrically (UV-1800 Shimadzu) at 305 nm for unentrapped drug. After the NLCs had precipitated at the bottom of the tube, they were collected and dried.
In Vitro Drug Release
In vitro drug release was performed using the dialysis bag method. The medium consisted of 5% (v/v) PEG400 in phosphate buffer at pH 7.4. For the release study, 2 mL of the NLC dispersion was placed into a dialysis sac (LA393-30MT dialysis membrane-70, Hi Media, Mumbai, India), which was then immersed into a 250-mL beaker acting as the receptor cell. The beaker contained 150 mL of the PEG400 5% (v/v) in phosphate buffer at pH 7.4 solution. The solution was agitated magnetically at 100 rpm, and the temperature was maintained at 37 ± 1°C. Samples were analyzed using a UV spectrophotometer (UV-1800 Shimadzu) at a wavelength of 321 nm. Drug concentration was determined using the standard curve prepared earlier.
Morphology Study
A transmission electron microscope (TEM 2100, JEOL, USA) was used to observe the shape of the prepared NLC. The NLC sample was prepared using negative staining method (1% uranyl acetate) and was further placed onto a sample holder and probed for observation (26).
Stability
Study of NLC
The stability study of the optimized NLC formulation was conducted by storing the formulation in tightly closed glass vials at 4°C, 50% RH, and 25°C, 65% RH, in the dark for a period of 3 months. The stability was evaluated monthly based on particle size, polydispersity index, entrapment efficiency, and drug release study in relation to storage time.
Statistical
Analysis
Measurements were made experimentally in duplicate for all data analysis. The data were presented as mean ± standard deviation (SD)/mean (SEM).
Result and Discussion
Yield of Mesua ferrea Seed Oil
The oil from M. ferrea seed was extracted using solvent extraction (Soxhlet method) using petroleum ether (b.p 40-60°C). Reddish brown-colored oil was extracted. The extracted oil's percentage yield was 70.93 ± 0.17% for the three successive extractions. Identification tests verify that the substance that was extracted is a lipid.
Physicochemical
Characteristics
The physicochemical parameters were determined using specific gravity, ash value, moisture content, acid value, saponification value, ester value, saponification value, iodine value, and peroxide value. The results found are shown in Table 2. The extracted M. ferrea L. oil’s physicochemical characteristics are almost identical to the other vegetable oils listed in USP (15).
Table 2. Physicochemical properties of the M. ferrea seed oil.
|
Characteristics |
Results |
|
Specific gravity |
0.93 ±1.19 |
|
Ash value |
3.5% ± 0.63% |
|
Moisture content |
3% ± 0.85% |
|
Acid value |
40.67 ± 1.24 |
|
Saponification value |
168.3 ± 1.06 |
|
Ester value |
127.63 ± 1.31 |
|
Unsaponifiable value (%) |
1.5 ± 0.25 |
|
Peroxide value |
3.5 ± 0.81 |
|
Iodine value |
70.11 ± 0.93 |
Characterization
of NLC
Particle Size, Size Distribution, and Zeta Potential
Particle size, polydispersity index, and zeta potential values are summarized in Table 3. The mean particle size ranged from 181.6 nm to 415.8 nm, with sizes below 200 nm enhancing bioavailability, making these NLCs suitable for cancer treatment (27). Both the solvent system and lipid composition influenced particle size. The acetone:isopropanol (8:2) solvent system produced smaller particles, consistent with studies showing that solvent mixtures like alcohol and dichloromethane enhance dissolution and bioavailability by reducing particle size (28). The lipid matrix containing Precirol ATO 5 also resulted in smaller particles (F8). The polydispersity indexes of the prepared NLCs ranged from 0.126 to 0.280, indicating that the NLCs were naturally polydispersed.
Table 3. Mean particle size, PDI, entrapment efficiency, drug loading, and zeta potential of the prepared NLCs.
|
Formulation code |
Particle size (nm) |
Polydispersity index |
Entrapment efficiency (%) |
Drug loading (%) |
Zeta potential |
|
F1 |
320.1±0.3 |
0.187±0.005 |
64±0.26 |
28.96±0.60 |
-14.8 |
|
F2 |
210.8±0.6 |
0.222±0.012 |
68.09±1.47 |
30.53±0.51 |
-11.9 |
|
F3 |
251±0.4 |
0.126±0.007 |
86.85±0.89 |
40.39±0.36 |
-30.8 |
|
F4 |
345±0.6 |
0.178±0.009 |
71.72±1.19 |
31.18±0.49 |
-17.6 |
|
F5 |
333.8±0.7 |
0.208±0.010 |
63.33±0.54 |
29.32±0.38 |
-22.4 |
|
F6 |
415.8±0.5 |
0.169±0.006 |
61.05±0.67 |
28.13±0.26 |
-23.1 |
|
F7 |
395.7±0.8 |
0.149±0.007 |
72±1.22 |
32.72±0.27 |
-28.1 |
|
F8 |
181.6±0.2 |
0.135±0.005 |
82.76±0.41 |
42.94±0.12 |
-30.1 |
|
F9 |
407.3±0.8 |
0.140±0.014 |
70.86±1.38 |
31.35±0.63 |
-26.3 |
The prepared NLCs exhibited a negative zeta potential ranging from -11.9 mV to -30.8 mV. This negative charge is attributed to Pluronic F68, a non-ionic surfactant that stabilizes the nanoparticles through steric stabilization, preventing aggregation and ensuring the colloidal stability of the NLCs (29). The negative zeta potential is an important indicator of colloidal stability, as it reduces the likelihood of particle aggregation by providing electrostatic repulsion between the negatively charged particles. Typically, particles are considered stable when their zeta potential is below -30 mV (30). All the optimized nanoformulations showed a high negative average zeta potential, although the variations in values can be attributed to differences in the solvent system and lipid mixture used in the formulation. Several studies support this, including research using a quality-by-design approach for NLC formulation, emphasizing lipid composition's critical role in determining zeta potential (31). Studies on valproic acid NLCs and other formulations have shown that the type and amount of lipids significantly affect the zeta potential, with optimized formulations achieving values around -24.4 mV, highlighting the sensitivity of this parameter to lipid selection (32).
Drug Entrapment Efficiency and Drug Loading
The entrapment efficiency and drug loading of the different formulations are presented in Table 3. The entrapment efficiency ranged from 61.05% to 82.76%, while drug loading ranged from 28.96% to 42.94%. It was observed that both entrapment efficiency and drug loading were influenced by the solubility of the drug in the solvent and lipid systems. Formulation F8 exhibited the highest entrapment efficiency (82.76%) and drug loading (42.94%), indicating that the formulation parameters for preparing the NLCs were optimal for drug incorporation. The high entrapment efficiency can be attributed to the excellent solubility of resveratrol within the oily core, which is a crucial component of the NLCs formulation (see Figure 1). For resveratrol, the NLCs create a novel nano-reservoir delivery system, where the drug is encapsulated within liquid compartments formed by a solid matrix. This suggests that M. ferrea oil is key in enhancing drug entrapment efficiency by providing an imperfect crystalline structure that facilitates drug encapsulation within the NLCs (33, 34).
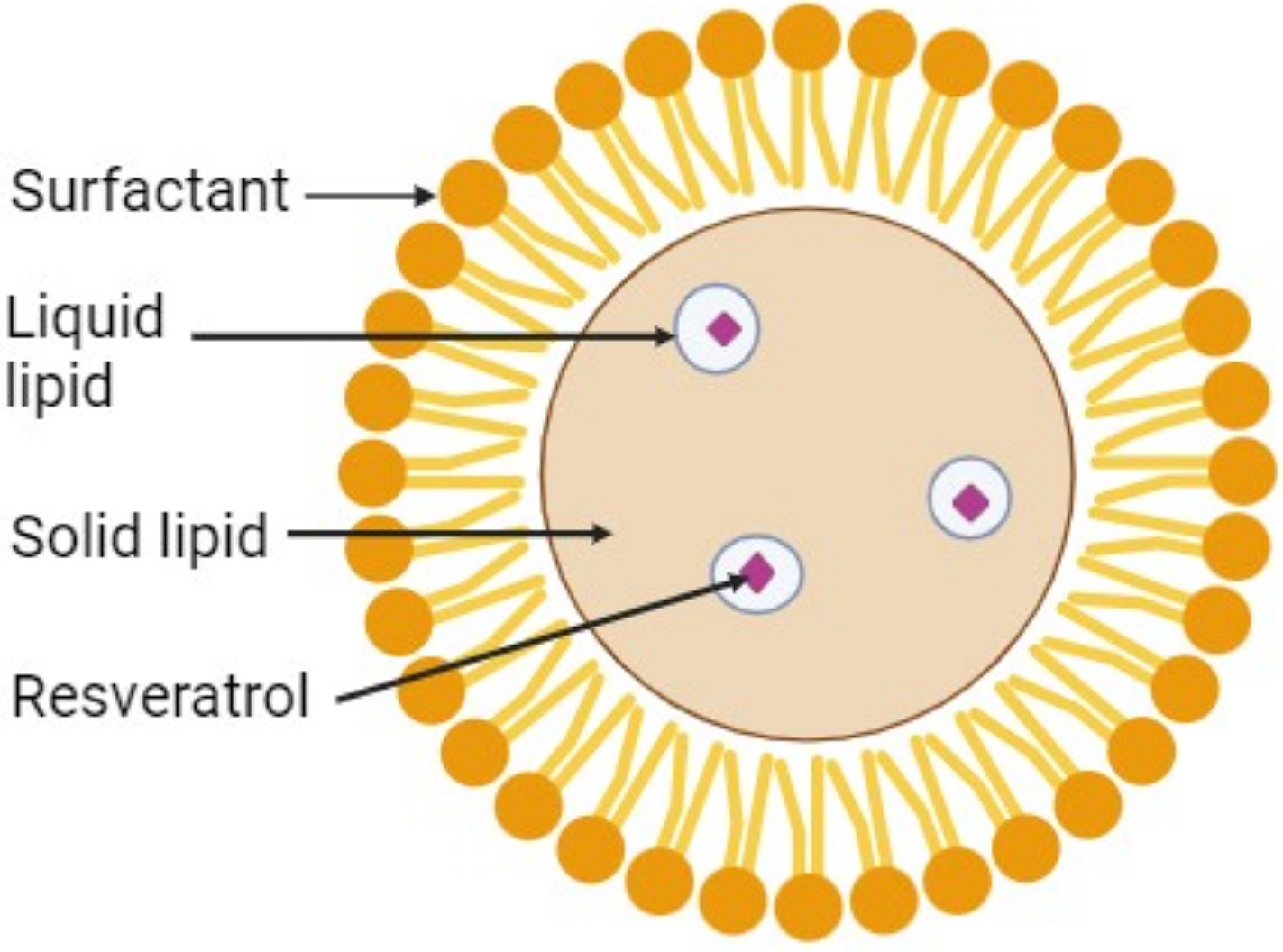
Figure 1. Illustration of resveratrol encapsulation in the oil core of NLC.
As shown in Table 3, formulation F8 demonstrated smaller particle size, higher entrapment efficiency, better drug loading, and a higher zeta potential. Therefore, formulation F8 was selected for further studies.
In Vitro Drug Release
The cumulative percentage release of resveratrol was evaluated using the dialysis bag method in phosphate buffer pH 7.4 with 5% PEG400 over 24 h. The results showed an initial burst release, followed by a sustained release, reaching up to 84.56% after 24 h (see Figure 2). The study indicated that the initial burst release of the drug from the nanoparticles (NLCs) is due to drug adsorption on the surface and the presence of liquid lipid in the outer shell. The drug dissolved in the liquid lipid is released rapidly during the initial phase, while the drug within the lipid matrix is released more gradually over time (26, 33). The optimized formulation (F8) exhibited the highest drug release among all the formulations.
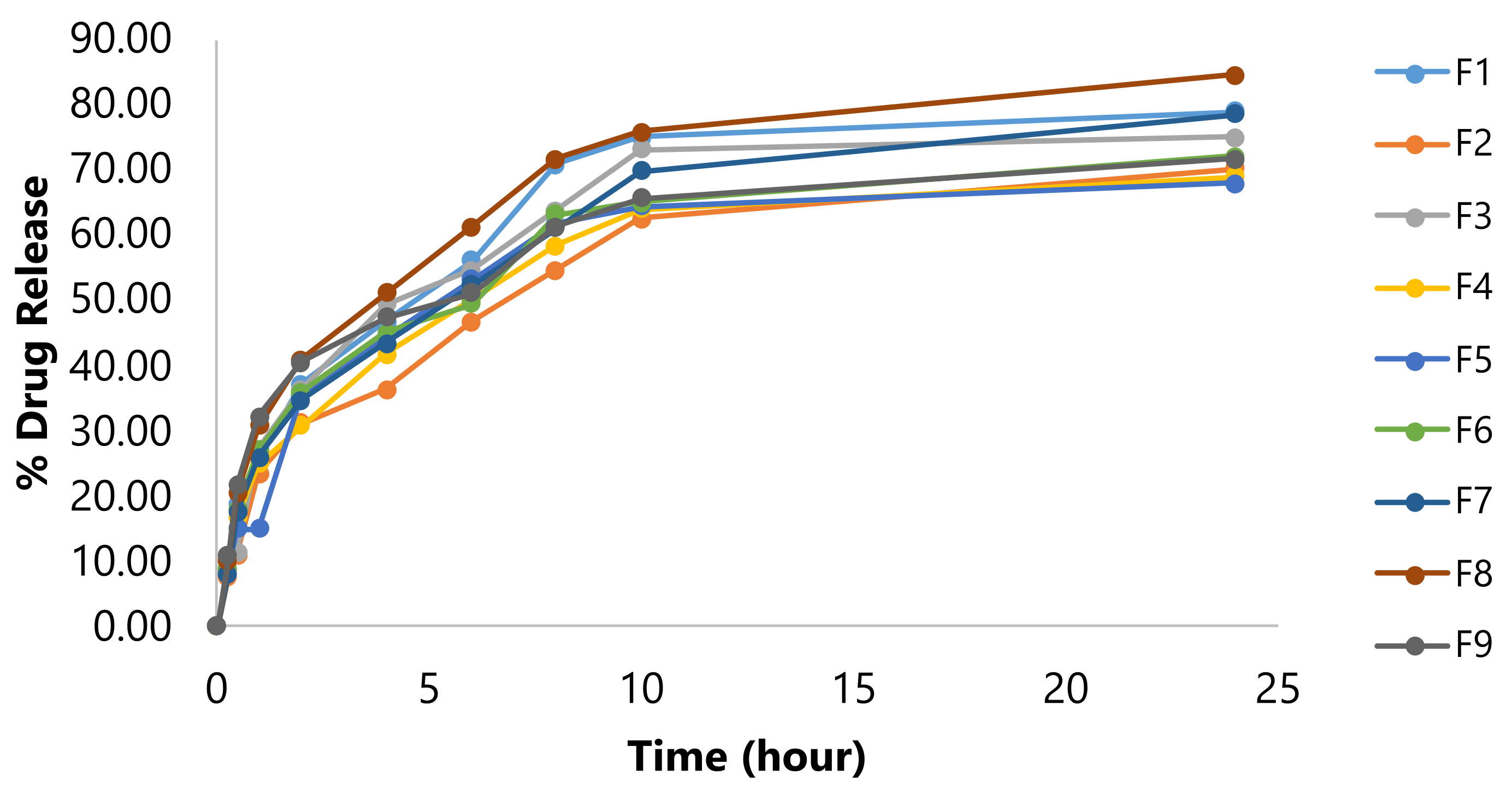
Figure 2. In vitro drug release of resveratrol from the prepared NLC.
Table 4. Stability study of the optimized formulation.
|
Storage time (months) |
4°C (50% RH) |
25°C (65% RH) |
||||
|
Mean particles diameter (nm) |
Polydispersity index |
Entrapment efficiency (%) |
Mean particles diameter (nm) |
Polydispersity index |
Entrapment efficiency (%) |
|
|
0 |
181.6±0.2 |
0.135±0.005 |
92.76±0.41 |
181.6±0.2 |
0.135±0.005 |
92.76±0.41 |
|
1 |
203.6±3.2 |
0.162±0.004 |
90.85±0.56 |
235.5±0.5 |
0.125±0.012 |
90.69±0.32 |
|
2 |
209.1±3.6 |
0.142±0.006 |
90.63±0.13 |
241.8±1.12 |
0.275±0.015 |
89.74±0.28 |
|
3 |
210.8±2.7 |
0.222±0.007 |
89.88±0.23 |
256.2±1.24 |
0.187±0.008 |
87.46±0.43 |
Morphology Study
The resveratrol-loaded NLCs displayed homogeneous, smooth-surfaced, spherical particles with uniform distribution and no aggregation, as confirmed by the low polydispersity index. The spherical shape results from the homogenization process, where the surfactant (Pluronic F-68) reduces the surface tension of the nanoparticles by minimizing interfacial energy. However, self-assembly requires external energy due to the steric or ionic repulsion between the hydrophobic and hydrophilic components, aiding nanoparticle formation and stabilization (35, 36). The TEM image of the optimized formulation is shown in Figure 3.
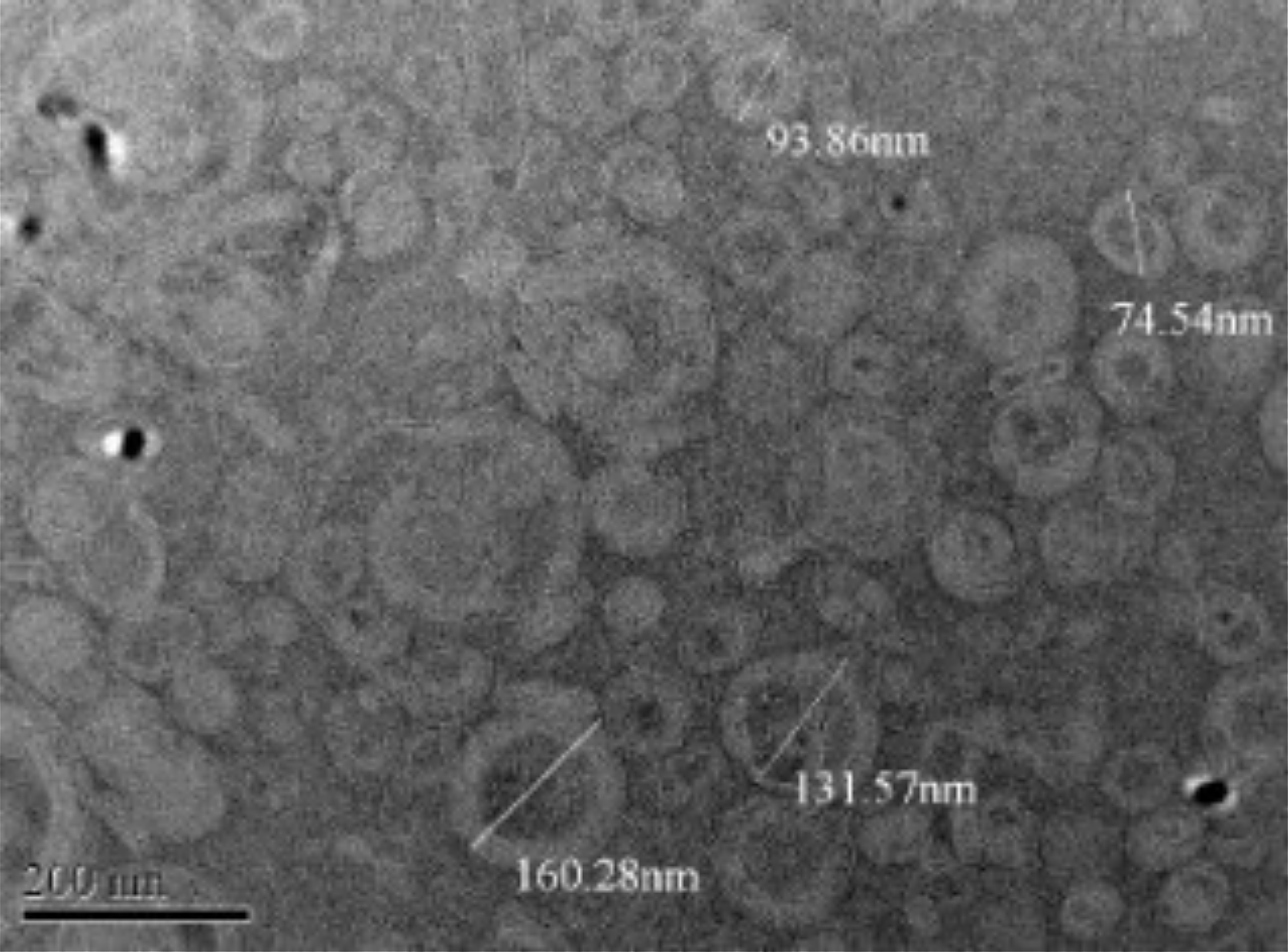
Figure 3. TEM image of resveratrol-loaded NLC.
Stability Study
For the stability study, the optimized NLC formulation (F8) was stored in the dark at 4°C, 50% RH, and 25°C, 65% RH for 3 months. The stability was assessed monthly based on particle size, polydispersity index, and entrapment efficiency. During the study, it was observed that no significant changes occurred in the mean particle size, polydispersity index, or entrapment efficiency of the NLCs when stored at 4°C. However, the particle size slightly increased at room temperature, and entrapment efficiency decreased. This may be due to the higher temperature (25°C), which increases the system's kinetic energy, potentially accelerating particle collisions and increasing the likelihood of particle aggregation. The results from the stability studies indicate that the NLCs are stable at refrigerated temperatures but less stable at room temperature (see Table 4).
Conclusion
This study reports the successful extraction and evaluation of M. ferrea seed oil as a pharmaceutical excipient. The prepared resveratrol-loaded NLCs, using M. ferrea seed oil, exhibited smaller, spherical, and homogeneous nanoparticles. The formulation containing an acetone-isopropanol solvent system (8:2) combined with M. ferrea oil and Precirol ATO 5 showed the best results in terms of particle size, zeta potential, entrapment efficiency, and drug release. Both entrapment efficiency and drug loading increased, indicating that the prepared NLCs were suitable for drug incorporation. The zeta potential and stability studies further confirmed the stability of the NLCs. The in vitro drug release pattern demonstrated an initial burst release, which can enhance drug penetration, followed by a controlled release for sustained delivery of the drug over an extended period. Based on these findings, it can be concluded that resveratrol-loaded NLCs have the potential to form homogeneous nanoparticles, which can enhance the incorporation of poorly water-soluble drugs and may offer greater efficacy than conventional resveratrol formulations for drug delivery. This study also provides a new perspective on the efficient use of M. ferrea seed oil in pharmaceutical nanoformulations.
Declarations
Ethics Statement
Not applicable.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The author declares no conflicting interest.
References
- Palaria B, Tiwari V, Tiwari A, Aslam R, Kumar A, Sahoo BM, et al. Nanostructured lipid carriers: A promising carrier in targeted drug delivery system. Current Nanomaterials. 2023;8(1):23-43.
- Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K, et al. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics. 2018;10(4).
- Dolatabadi S, Karimi M, Nasirizadeh S, Hatamipour M, Golmohammadzadeh S, Jaafari MR. Preparation, characterization and in vivo pharmacokinetic evaluation of curcuminoids-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). Journal of Drug Delivery Science and Technology. 2021;62:102352.
- Viegas C, Patrício AB, Prata JM, Nadhman A, Chintamaneni PK, Fonte P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics. 2023;15(6).
- Patel D, Dasgupta S, Dey S, Ramani YR, Ray S, Mazumder B. Nanostructured Lipid Carriers (NLC)-Based Gel for the Topical Delivery of Aceclofenac: Preparation, Characterization, and In Vivo Evaluation. Scientia of Pharmaceutica. 2012;80(3):749-764.
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Advanced Pharmaceutical Bulletin. 2015;5(3):305–313.
- Eleraky NE, Omar MM, Mahmoud HA, Abou-Taleb HA. Nanostructured Lipid Carriers to Mediate Brain Delivery of Temazepam: Design and In Vivo Study. Pharmaceutics. 2020;12(5).
- Iqbal B, Ali J, Ganguli M, Mishra S, Baboota S. Silymarin-loaded nanostructured lipid carrier gel for the treatment of skin cancer. Nanomedicine (Lond). 2019;14(9):1077-1093.
- Neves AR, Lúcio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. International Journal of Nanomedicine. 2013;8:177-187.
- Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharmaceutical Journal. 2021;29(9):999-1012.
- Khan S, Sharma A, Jain V. An overview of nanostructured lipid carriers and its application in drug delivery through different routes. Advanced Pharmaceutical Bulletin. 2022;13(3):446.
- Sruthikrishna P. Review on ethnobotany and phytopharmacology on Mesua ferrea Linn. Research Journal of Pharmacognosy and Phytochemistry. 2021;13(4):195-9.
- Kumar D, Arya V, Kaur R, Bhat ZA, Gupta VK, Kumar V. A review of immunomodulators in the Indian traditional health care system. Journal of Microbiology, Immunology and Infection. 2012;45(3):165-184.
- Patangia U, Wal A, Gupta D, Singh I, Wal P. A review of the phytochemical constituents and pharmacological activities of Nagkesar (Mesua ferrea Linn). Traditional Medicine Research. 2023;8(3):14.
- Tapash Chakraborty MKD. Oil of Mesua ferrea L. Seed as a Promising Pharmaceutical Excipient in Lipid Based Nanoformulation. 2017;7(7). 133-141.
- da Rocha Lindner G, Khalil NM, Mainardes RM. Resveratrol-loaded polymeric nanoparticles: validation of an HPLC-PDA method to determine the drug entrapment and evaluation of its antioxidant activity. Scientific World Journal. 2013;2013:506083.
- Annaji M, Poudel I, Boddu SH, Arnold RD, Tiwari AK, Babu RJ. Resveratrol‐loaded nanomedicines for cancer applications. Cancer Reports. 2021;4(3):e1353.
- Zheng Y, Jia R, Li J, Tian X, Qian Y. Curcumin-and resveratrol-co-loaded nanoparticles in synergistic treatment of hepatocellular carcinoma. Journal of Nanobiotechnology. 2022;20(1):339.
- Lin M, Yao W, Xiao Y, Dong Z, Huang W, Zhang F, et al. Resveratrol-modified mesoporous silica nanoparticle for tumor-targeted therapy of gastric cancer. Bioengineered. 2021;12(1):6343-6353.
- Singh G, Pai RS. Optimized PLGA nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opinion on Drug Delivery. 2014;11(5):647-659.
- Saonere JA, Channawar MA, Kochar NI, Mohale D, Chandewar AV. Pharmacognostic evaluation and In-vitro evaluation of antioxidant activity of Mesua ferrea Linn. Research Journal of Pharmacy and Technology. 2023;16(9):4234-8.
- De A, De S, Saha N, Das B, Naskar S, Samanta A. Pharmacopoeias, national formulary and extra pharmacopoeia. Dosage Forms, Formulation Developments and Regulations: Elsevier; 2024. p. 83-98.
- Pulingam T, Foroozandeh P, Chuah J-A, Sudesh K. Exploring various techniques for the chemical and biological synthesis of polymeric nanoparticles. Nanomaterials. 2022;12(3):576.
- Gu X, Zhang W, Liu J, Shaw JP, Shen Y, Xu Y, et al. Preparation and characterization of a lovastatin-loaded protein-free nanostructured lipid carrier resembling high-density lipoprotein and evaluation of its targeting to foam cells. AAPS PharmSciTech. 2011;12(4):1200-1208.
- Gomaa E, Fathi HA, Eissa NG, Elsabahy M. Methods for preparation of nanostructured lipid carriers. Methods. 2022;199:3-8.
- Das J, Lahan M, Bharali A, Ghose S, Sahu BP, Laloo D, et al. Enhancing resveratrol pharmacokinetics and cytotoxicity in ovarian cancer cells via nanostructured lipid carriers. Journal of Dispersion Science and Technology. 2024:1-12.
- Akanda M, Getti G, Douroumis D. In vivo evaluation of nanostructured lipid carrier systems (NLCs) in mice bearing prostate cancer tumours. Drug Delivery and Translational Research. 2021:1-13.
- Ha E-S, Park H, Lee S-K, Sim W-Y, Jeong J-S, Baek I-h, et al. Pure trans-resveratrol nanoparticles prepared by a supercritical antisolvent process using alcohol and dichloromethane mixtures: effect of particle size on dissolution and bioavailability in rats. Antioxidants. 2020;9(4):342.
- Zhao S, Yang X, Garamus VM, Handge UA, Bérengère L, Zhao L, et al. Mixture of nonionic/ionic surfactants for the formulation of nanostructured lipid carriers: effects on physical properties. Langmuir. 2014;30(23):6920–6928.
- Ong YS, Banobre-Lopez M, Lima SAC, Reis S. A multifunctional nanomedicine platform for co-delivery of methotrexate and mild hyperthermia towards breast cancer therapy. Materials Science and Engineering: C. 2020;116:111255.
- Bagde S, Mamidi HK. Nanostructured Lipid Carriers of Eperisone Hydrochloride using Quality By Design Approach. Journal of Drug Delivery and Therapeutics. 2023;13(2):37-46.
- Varshosaz J, Eskandari S, Tabakhian M. Production and optimization of valproic acid nanostructured lipid carriers by the Taguchi design. Pharmaceutical Development and Technology. 2010;15(1):89-96.
- Kim CH, Kang TH, Kim BD, Lee TH, Yoon HY, Goo YT, et al. Enhanced docetaxel delivery using sterically stabilized RIPL peptide-conjugated nanostructured lipid carriers: In vitro and in vivo antitumor efficacy against SKOV3 ovarian cancer cells. International Journal of Pharmaceutics. 2020;583:119393.
- Poonia N, Kaur Narang J, Lather V, Beg S, Sharma T, Singh B, et al. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: Systematic development, characterization and pharmacokinetic evaluation. Colloids and Surfaces B: Biointerfaces. 2019;181:756-766.
- Elattar GS, Aboutaleb AE-S, Abdel-Rahman AA-Z, Tawfeek HM. Investigation of the Impact of Different Formulation and Processing Parameters on the Physicochemical Properties of Curcumin-Loaded Nanostructured Lipid Carriers. Sphinx Journal of Pharmaceutical and Medical Sciences. 2024;7(1):9-20.
- Ortiz AC, Yañez O, Salas-Huenuleo E, Morales JO. Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics. 2021;13(4):531.

 ETFLIN
Notification
ETFLIN
Notification






