Praecitrullus fistulosus Fruit Extract Ameliorates Type II Diabetic Complications in Rats: In Silico, In Vitro, and In Vivo Investigation
by Shanti Bhushan Mishra ★ , Juhi Verma, Garima Sahu, Nishi Gupta
Academic editor: Garnadi Jafar
Sciences of Pharmacy 4(1): 1-8 (2025); https://doi.org/10.58920/sciphar0401291
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
15 Oct 2024
28 Dec 2024
06 Jan 2025
13 Jan 2025
Abstract: This study explored the safety and antidiabetic potential of a hydroalcoholic extract of Praecitrullus fistulosus fruits, along with qualitative and quantitative phytochemical analyses. The antidiabetic effect was evaluated using in vitro methods, including α-amylase and α-glucosidase inhibition assays, as well as an in vivo high-fat diet and low-dose streptozotocin-induced diabetic model. Molecular docking studies were conducted to identify phytochemicals responsible for the antidiabetic effects. The fruit extract exhibited maximum inhibition of 52.06% and 58.10% for α-amylase and α-glucosidase enzymes, respectively, at a concentration of 100 µg/mL. The extract also demonstrated a significant (p < 0.001) and dose-dependent antidiabetic effect at oral doses of 200 mg/kg and 400 mg/kg in the tested animals. In silico analysis revealed that α-tocopherol exhibited the best docking pose, with a docking energy of -8.2 kcal/mol. Based on the results, it can be concluded that the hydroalcoholic extract of Praecitrullus fistulosus contains phytochemicals effective in controlling glucose levels. This study also validates the traditional use of Praecitrullus fistulosus fruits in managing diabetes.
Keywords: Amylolytic enzymesα-amylaseα-glucosidaseMolecular docking
Introduction
Diabetes mellitus is a group of metabolic disorders characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Insufficient insulin activity on target tissues leads to impaired metabolism of carbohydrates, proteins, and lipids, which manifests in symptoms such as polyuria, polydipsia, and polyphagia (1, 2). In type 2 diabetes mellitus (T2DM), insulin resistance in tissues such as the liver, adipose tissue, and muscles, combined with pancreatic β-cell dysfunction, disrupts the feedback loop between insulin sensitivity and insulin secretion. This results in erratic blood glucose levels, reduced glucose uptake in peripheral tissues, and increased hepatic glucose production (3). The global prevalence of diabetes is projected to rise from 4% in 1995 to 6.4% by 2025, with type 2 diabetes accounting for 85–95% of cases in industrialized nations and an even higher percentage in developing countries. Approximately 80% of all diabetes patients reside in emerging economies, with India and China experiencing the highest burden. The growing incidence of diabetes and its associated complications has intensified the search for effective and safer anti-hyperglycemic agents (4).
Current treatment options for T2DM include insulin therapy and several classes of oral anti-diabetic drugs, such as biguanides, sulfonylureas, glinides, and amylolytic enzyme inhibitors. While these medications are effective in controlling blood glucose levels, their use is often limited by adverse effects and insufficient long-term efficacy. Consequently, researchers are increasingly turning to medicinal plants as sources of novel therapeutic agents. Plants have been utilized for centuries in traditional medicine due to their natural origins and minimal side effects. Many medicinal plants and their extracts have demonstrated efficacy in managing diabetes, making them attractive alternatives to conventional treatments (5). One such plant is Praecitrullus fistulosus, commonly known as "Tinda" in Hindi, which belongs to the Cucurbitaceae family. This plant, native to northwest India, is widely cultivated in India, Afghanistan, and Pakistan as a vegetable. Cucurbits are renowned for their nutritional and medicinal properties, containing bioactive compounds essential for human health (6). Previous studies have identified various phytochemicals in P. fistulosus, including flavonoids, polyphenols, alkaloids, tannins, phytosterols, and cardiac glycosides (7). These compounds contribute to its reported pharmacological activities, such as antimicrobial, anthelmintic, free radical scavenging, and anti-diabetic effects.
To explore the potential of P. fistulosus as a natural remedy for diabetes mellitus, this study investigates its anti-diabetic properties using in silico, in vitro, and in vivo approaches. By evaluating its bioactive compounds and mechanisms of action, this research aims to provide scientific validation for the traditional use of P. fistulosus in managing diabetes (8, 9).
Materials and
Methods
Plant Material Collection and Authentication
Fresh, mature fruits of P. fistulosus were collected from local areas in Prayagraj in August 2022. The plant was taxonomically identified by experts at the National Institute of Science Communication and Information Resources (NISCAIR), New Delhi. A voucher specimen was prepared and submitted to the Raw Materials Herbarium and Museum (RHMD), New Delhi, under the verification number NISCPR/RHMD/Consult/2023/4502- 03.
Preparation of Extract
The collected fruits were thoroughly washed, air-dried, and ground into a fine powder. The powdered material was subjected to extraction using 50% ethanol as a solvent through the Soxhlet extraction method. The resulting extract was concentrated by evaporating the solvent at a controlled temperature of 40–50 °C using a rotary evaporator under reduced pressure (10).
Quantitative Phytochemical Tests
Total Phenolic Content
The total phenolic content (TPC) in the ethanolic extract of P. fistulosus (PFEE) was determined using the Folin-Ciocalteu reagent, following the methods described by Chandra et al. (2014) and Harborne (1998) (11, 12). Gallic acid was used as the reference standard. The absorbance of the sample was measured at 765 nm using a UV-visible spectrophotometer. The TPC was calculated in terms of milligrams of gallic acid equivalent per gram of extract (mg GAE/g) based on a standard calibration curve.
Total Flavonoid Content
The total flavonoid content (TFC) of the PFEE was estimated using the method described by Sembiring et al. (2018) (13). Quercetin was used as the reference standard. The absorbance of the sample was measured at 510 nm using a UV-visible spectrophotometer. The TFC was calculated based on a standard calibration curve and expressed as milligrams of quercetin equivalent per gram of extract (mg QE/g).
Molecular Docking
The objective of this study was to investigate the binding interactions of PFEE constituents with human pancreatic α-amylase. The target protein molecule was retrieved from the Protein Data Bank (PDB) with the identifier 1XEW at a resolution of 2.000 Å. Preparation of the target protein for docking involved removing all heteroatoms, non-receptor atoms, and water molecules.
Constituents of PFEE, including α-tocopherol, linoleic acid, myristic acid, oleanolic acid, palmitic acid, and ursolic acid, were modeled and optimized using ChemDraw Ultra 16.0 software to generate appropriate ligand structures. These ligands were then subjected to in silico docking simulations to estimate binding energies and determine their optimal docked configurations. The docking studies were performed using the Mcule platform (14).
In Vitro Antidiabetic Activity
Assay of α-Amylase Inhibition
The assay was conducted using substrates containing 0.01 M CaCl₂ (0.2 mL), 2 mg of starch, and 1 M Tris-HCl buffer (pH 7.2). The substrate solution was pre-incubated at 37 °C for 5 min and then boiled for 5 min. The PFEE was dissolved in DMSO at various concentrations (20, 40, 60, 80, and 100 µg/mL) and added to the substrate solution. Subsequently, 0.1 mL of α-amylase (diluted to 2 units/mL) was introduced, and the mixture was incubated for 10 min at 37 °C.
The enzymatic reaction was terminated by adding 0.5 mL of 50% acetic acid to each test tube. The solution was then centrifuged at 3000 rpm for 5 min at 4 °C, and the absorbance of the supernatant was measured at 595 nm using a spectrophotometer. Acarbose, a known amylolytic enzyme inhibitor, was used as the reference standard. All tests were performed in triplicate for each concentration, and the results were calculated using the Equation 1 (15, 20).
Equation 1
where, As = Absorbance of control and At = Absorbance of test samples at 595 nm.
Assay of α-Glucosidase Inhibition
The assay was performed using a 100 mL solution of phosphate buffer (pH 6.8) containing 1 mg of α-glucosidase. For each test, 200 µL of α-glucosidase solution was combined with 100 µL of PFEE at concentrations of 20, 40, 60, 80, and 100 µg/mL. To initiate the reaction, 100 µL of 3 mM p-nitrophenyl-D-glucopyranoside (p-NPG) was added to each test tube, and the mixtures were incubated at 37 °C for 20 min.
The enzymatic reaction was terminated by adding 2 mL of 0.1 M Na₂CO₃ to each test tube. The activity of α-glucosidase was determined by measuring the absorbance of the reaction product at 405 nm using a UV-visible spectrophotometer. Acarbose, a known amylase and glucosidase inhibitor, was used as the positive control (16).
In Vivo Studies
Animals
Adult Wistar rats (weighing 100–250 g) were procured from an animal supplier approved by the Committee for Control and Supervision of Experiments on Animals (CPCSEA), M/s Chakraborty Enterprises, Kolkata (registration number 1443/PO/Bt/s/11CPCSEA). The animals were transferred to the quarantine area of the animal house at the United Institute of Pharmacy, where they were acclimatized for two weeks before the experiment. During this period, the rats were provided with food and water ad libitum and maintained under a controlled environment with a 12-hour light/dark cycle. The experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) under approval number UIP/IAEC/March-2023/03.
Acute Oral Toxicity
The determination of the median lethal dose (LD₅₀) was conducted in accordance with OECD Guideline 423. The extract was administered to animals at doses of 5, 50, 300, and 2000 mg/kg, as specified in the guidelines. Following administration, the animals were closely monitored for any signs of toxicity, behavioral changes, or adverse effects (17, 19).
Antidiabetic Activity
All animals were fed a high-fat diet (HFD) for the first 13 days. On the 13th day, streptozotocin (STZ) was administered intraperitoneally (I.P.) at a dose of 40 mg/kg body weight to all animals except those in the normal control group (18). Blood glucose levels were measured 72 h post-STZ administration. Animals with blood glucose levels ≥ 200 mg/dL were classified as diabetic and included in the experiment. The animals were divided into five groups, each comprising six animals:
- Group 1 (Normal Control): Received normal food and water.
- Group 2 (Diabetic Control): Received HFD and STZ (40 mg/kg body weight).
- Group 3 (Standard Treatment): Treated with metformin (100 mg/kg body weight).
- Group 4 (Low Dose PFEE): Treated with a low dose of PFEE at 200 mg/kg body weight.
- Group 5 (High Dose PFEE): Treated with a high dose of PFEE at 400 mg/kg body weight.
All treatments, including the vehicle, standard drug, and plant extracts, were administered orally using an oral gavage tube. Blood samples were collected weekly via retro-orbital puncture, and blood glucose levels were measured using a glucometer (Morepen). Additional blood samples collected through retro-orbital puncture were used for lipid profile analysis. Lipid parameters, including low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), triglycerides (TG), and total cholesterol (TC), were assessed using standard commercial kits (Erba Mannheim, Mumbai, India).
Statistical Analysis
Graph pad prism 9.5.1 was applied to conduct the statistical testing. All the information is presented as Mean ± SD. The two-way ANOVA model with Tukey’s multiple comparisons test was used to examine body weight, blood glucose, and lipid profile.
Results
Phytochemical
Screening
The results of the quantitative phytochemical analysis revealed that the TPC of PFEE was 250.56 mg/g, expressed as vanillic acid equivalents (VAE). The TFC was determined to be 273.18 mg/g, expressed as quercetin equivalents (QE) on a dry weight basis.
Molecular Docking
Acarbose and different constituents of PFEE like α-tocopherol, linoleic acid, myristic acid, oleanolic acid, palmitic acid, and ursolic acid were involved in effective binding relationships with the target molecule as shown in Table 1 and Figure 1.
In Vitro Antidiabetic Activity
α-Amylase and α-Glucosidase Inhibitory Activity
The anti-diabetic potential of PFEE was evaluated by assessing its inhibitory effects on two key amylolytic enzymes, α-amylase and α-glucosidase, in comparison with the standard drug acarbose. Acarbose demonstrated 65.28% inhibition of α-amylase (Figure 2A) and 69.38% inhibition of α-glucosidase (Figure 2B) at a concentration of 100 µg/mL. At the same concentration, PFEE exhibited 52.06% inhibition of α-amylase and 58.10% inhibition of α-glucosidase. Both α-amylase and α-glucosidase play crucial roles in the digestion of carbohydrates, making them important targets for managing postprandial hyperglycemia in type 2 diabetes.Acarbose, a widely used oral hypoglycemic agent, lowers blood sugar by inhibiting these saccharide-hydrolyzing enzymes. Similarly, PFEE demonstrated inhibitory activity against both enzymes at varying concentrations, suggesting its potential as a natural alternative to conventional enzyme inhibitors.
Table 1. Interaction of constituents of PFEE target α-amylase.
|
Constituents of PFEE |
Hydrogen Bonds with Target |
|
Acarbose |
GLN302, ASN301, ALA307, HIS305, ASP |
|
-tocopherol |
GLY304, GLN302, ARG303, THR163, ASP300, ASN301, HIS201, LEU165, GLY74, LEU32 |
|
Linoleic acid |
VAL98, ALA97, LEU196, ALA198 |
|
Myristic acid |
LEU196, ASP197, ALA198 |
|
Oleanolic acid |
ASP300, HIS305, ASN301, ARG56, GLN302, ALA57 |
|
Palmitic acid |
LEU196, ALA197, ASP197, ASP196, ARG195, ALA198 |
|
Ursolic acid |
LEU26, ARG303, GLY304, GLY308, ALA33, HIS305, THR84, ASP300 |
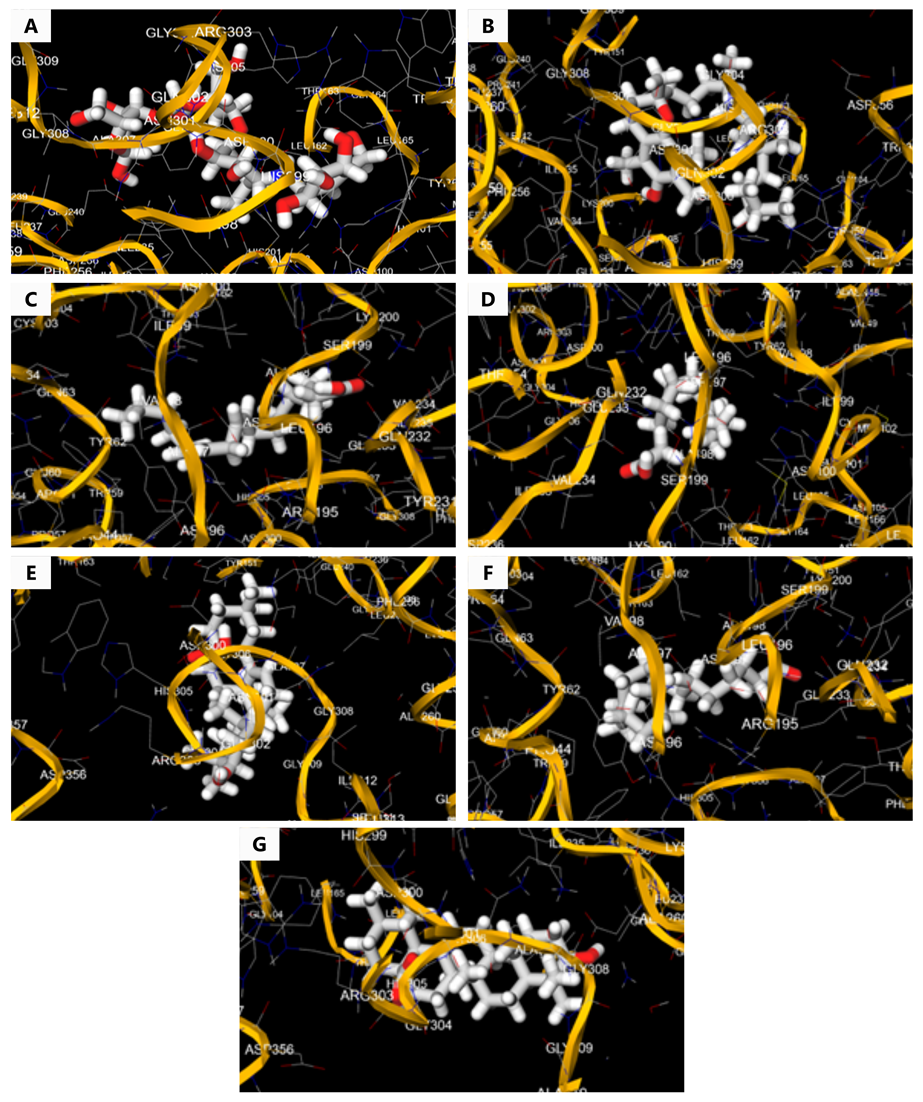
Figure 1. 3D interaction of α-amylase with (A) acarbose, (B) α-tocopherol, (C) linoleic acid, (D) myristic acid, (E) oleanolic acid, (F) palmitic acid, and (G) ursolic acid.
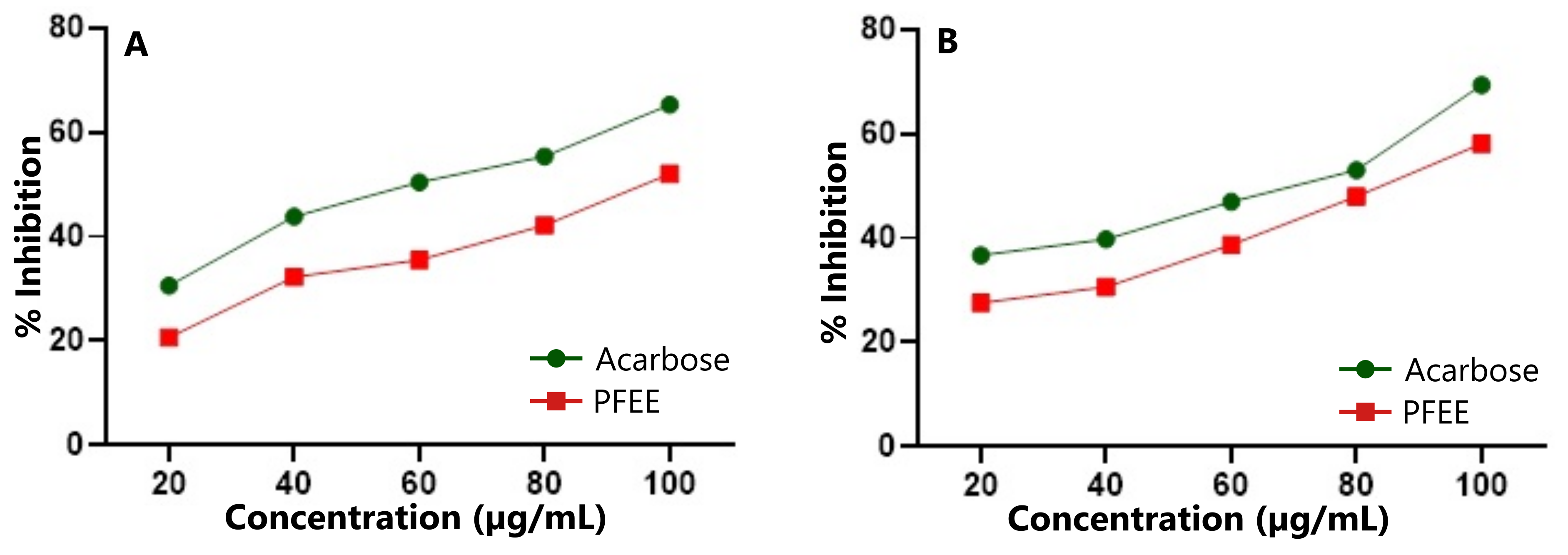
Figure 2. Inhibition effect of PFEE on (A) α-amylase and (B) α-glucosidase.
In Vivo Studies
Oral Acute Toxicity
In the oral acute toxicity study, the PFEE was administered at doses of 5, 50, 300, and 2000 mg/kg body weight to assess its safety profile. Throughout the study, no mortality or significant adverse effects were observed at any of the tested doses, indicating that the extract is safe for use within the evaluated dosage range.
Effect of PFEE on Blood Glucose Level
Oral administration of PFEE at doses of 200 mg/kg and 400 mg/kg resulted in a significant reduction in blood glucose levels over 39 days, as shown in Figure 3A. After the treatment period, PFEE at a dose of 200 mg/kg reduced glucose levels from 222.16 mg/dL to 115 mg/dL. A higher dose of 400 mg/kg resulted in a more pronounced reduction, from 222.16 mg/dL to 105.5 mg/dL. For comparison, the standard drug metformin, administered at 100 mg/kg, reduced glucose levels from 222.16 mg/dL to 98.16 mg/dL.
In comparison to the diabetic control group, all treated groups exhibited a significant (p < 0.0001) dose-dependent decrease in blood glucose levels. These results demonstrate the potential of PFEE as an effective agent for lowering blood glucose levels in diabetic rats.
Effect of PFEE on Lipid Profile
During the experimental study, various lipid parameters, including high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), total cholesterol (TC), and triglycerides (TG), were evaluated to assess the efficacy of PFEE. As shown in Figure 3B, PFEE at a dose of 400 mg/kg had a significant impact on the lipid profile of diabetic rats.
PFEE treatment led to a marked reduction in TC levels from 148 ± 12.26 mg/dL to 78.33 ± 4.71 mg/dL (p < 0.0001), TG levels from 108.5 ± 6.47 mg/dL to 81.33 ± 4.81 mg/dL, LDL levels from 54.16 ± 8.91 mg/dL to 32.5 ± 6.21 mg/dL, and VLDL levels from 37.16 ± 5.59 mg/dL to 25.33 ± 2.74 mg/dL. Additionally, HDL levels were significantly increased from 17.83 ± 6.71 mg/dL to 23.5 ± 4.92 mg/dL compared to the diabetic control group.
Discussion
This study investigated the antidiabetic effect of PFEE using a rodent model combining a high-fat diet (HFD) and low-dose streptozotocin (STZ). HFD induces insulin resistance through mechanisms like the glucose-fatty acid cycle (Randle cycle) (21, 22), while low-dose STZ impairs insulin secretion, mimicking late-stage type 2 diabetes mellitus (23). Diabetic rats exhibited elevated fasting glucose levels, indicating insulin resistance. This model effectively simulates human metabolic conditions, including hyperglycemia, insulin resistance, and hyperlipidemia, making it highly relevant for studying diabetes-related pathophysiology (24).
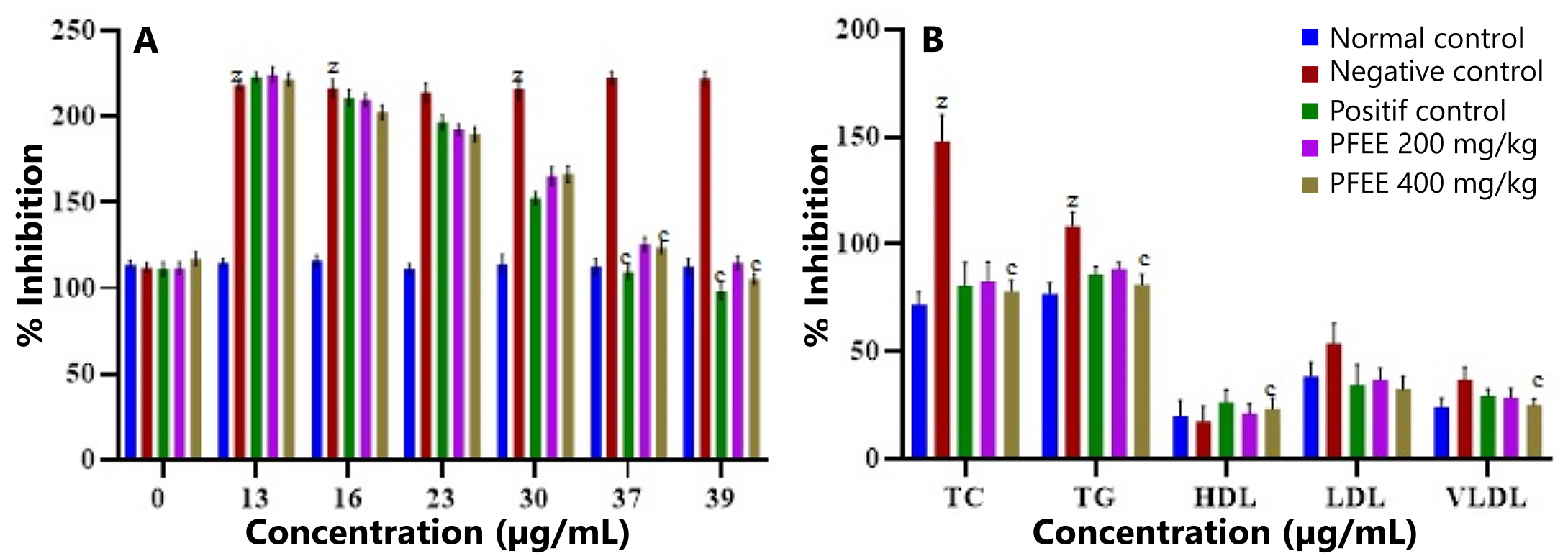
Figure 3. Effect of PFEE on (A) glucose level and (B) lipid profile. (c, p < 0.0001) compared to the diabetic control group and (z, p < 0.0001) compared to the normal control group.
The fruits of P. fistulosus are rich in phytochemicals such as flavonoids, terpenoids, phenols, and other secondary metabolites, which contribute to its potential as an anti-hyperglycemic agent (25, 26). In this research, the total phenolic and flavonoid contents of PFEE were quantified, and the observed anti-diabetic effects were attributed to the presence of polyphenols and flavonoids. These compounds act as free radical scavengers, reducing glucose absorption, enhancing glucose uptake in peripheral tissues, and regulating key metabolic pathways such as glycolysis and glycogen synthesis (27).
Molecular
Docking
In the molecular docking analysis, α-tocopherol exhibited the most favorable binding interaction with α-amylase (PDB ID: 1XEW), forming hydrogen bonds with residues GLY304, GLN302, ARG303, THR163, ASP300, ASN301, HIS201, LEU165, GLY74, and LEU32. The docking energy for this interaction was -8.2 kcal/mol. Ursolic acid also demonstrated strong binding, interacting with residues LEU26, ARG303, GLY304, GLY308, ALA33, HIS305, THR84, and ASP300, with a docking energy of -5.6 kcal/mol. These phytoconstituents are known for their roles in managing type 2 diabetes and oxidative stress in metabolic disorders (25, 26).
In Vitro
Enzyme Inhibition
The in vitro anti-diabetic potential of PFEE was evaluated using α-amylase and α-glucosidase inhibition assays, with acarbose as the standard. Both enzymes play key roles in carbohydrate digestion and postprandial glucose elevation. Inhibiting these enzymes can reduce postprandial hyperglycemia and lower the risk of developing diabetes (23). Phenolic acids and flavonoids, the dominant polyphenolic constituents in PFEE, have demonstrated significant inhibitory effects on amylolytic enzymes (28). The presence of these compounds in PFEE supports its ability to inhibit these enzymes at different concentrations, thereby reducing blood glucose levels in diabetic rats, as observed in comparison with acarbose.
Acute
Toxicity and Safety Profile
In the oral acute toxicity study, PFEE was administered at doses up to 2000 mg/kg body weight. No signs of toxicity or mortality were observed during the observation period, indicating an LD₅₀ greater than 2000 mg/kg. These findings confirm the extract's safety at the tested doses.
Dose-Dependent
Effects on Glycemic and Lipid Profiles
The study also observed that PFEE exhibited dose-dependent effects, with higher doses producing greater reductions in fasting blood glucose levels. This effect is likely due to the higher concentration of active constituents in the extract at increased doses. PFEE also ameliorated lipid abnormalities, including reductions in cholesterol and triglyceride levels, along with an increase in HDL levels. These improvements in lipid metabolism suggest enhanced insulin activity.
The flavonoids present in PFEE may play a key role in these effects. Flavonoids are known to inhibit cholesterol absorption, enhance triglyceride-laden lipoprotein catabolism, and promote bile acid excretion. Additionally, they can inhibit enzymes such as HMG-CoA reductase, reducing cholesterol synthesis, and cholesterol acyltransferase, decreasing cholesterol esterification in the liver and intestines. These mechanisms collectively contribute to reduced cholesterol absorption and incorporation into lipoproteins (29).
Conclusion
In the Cucurbitaceae family, P. fistulosus is one of the potent plants containing essential phytoconstituents that promote human health. The entire plant and its fruit are considered natural antioxidant storehouses with significant therapeutic potential for combating various diseases. The present study demonstrated the antidiabetic potential of P. fistulosus in STZ and high-fat diet-induced diabetic animal models, with no observed side effects or toxicity. Blood glucose levels decreased from 222.16 mg/dL to 115 mg/dL at a dose of 200 mg/kg bw (p < 0.0001). Additionally, molecular docking research supports the development of this plant as a potential diabetic medication, showing positive interactions with constituents responsible for the antiglycation activity. In vitro results also confirmed that the extract's inhibition of amylolytic enzymes in the intestine could contribute to its antidiabetic and antihyperlipidemic effects. Our findings suggest that P. fistulosus could be considered a promising natural remedy for the treatment of diabetes and its related complications.
Declarations
Acknowledgment
The authors greatly acknowledge and express their gratitude to the Principal United Institute of Pharmacy, Prayagraj for providing instruments and laboratory and animal house facilities to carry out this research work.
Ethics Statement
The experimental procedure was approved by the IAEC committee with approval number (UIP/IAEC/March-2023/03).
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Association AD, Diagnosis and classification of diabetes mellitus, Diabetes care, 2010; 33 (Supplement_1): S62-S9.
- Ramachandran A, Know the signs and symptoms of diabetes, The Indian journal of medical research, 2014; 140 (5): 579.
- Zheng Y, Ley SH, Hu FB, Global aetiology and epidemiology of type 2 diabetes mellitus and its complications, Nature reviews endocrinology, 2018; 14 (2): 88-98.
- Zimmet PZ, Magliano DJ, Herman WH, Shaw JE, Diabetes: a 21st century challenge, The lancet Diabetes & endocrinology, 2014; 2 (1): 56-64.
- Rathinavelusamy P, Mazumder PM, Sasmal D, Jayaprakash V, Evaluation of in silico, in vitro α-amylase inhibition potential and antidiabetic activity of Pterospermum acerifolium bark, Pharmaceutical Biology, 2014; 52 (2): 199-207.
- Verma A and Kumar V, Trends in hybrid cucumber development, Vegetable Science, 2020; 47(2): 274-284.
- Kalpesh B. Ishnava, Patel KS, In vitro study of Praecitrulus fistulosus (Stocks) Pangalo (Cucurbitaceae) fruit – A potential candidate of Anthelmintic activity, Bulletin of the National Research Centre, 2020; 130: 1-10.
- Shivamadhu MC, Srinivas BK, Jayarama S, Chandrashekaraiah SA, Anti-cancer and anti-angiogenic effects of partially purified lectin from Praecitrullus fistulosus fruit on in vitro and in vivo model, Biomedicine & Pharmacotherapy, 2017; 96: 1299-309.
- Sofowora A, Ogunbodede E, Onayade A, The role and place of medicinal plants in the strategies for disease prevention, African journal of traditional, complementary and alternative medicines, 2013; 10 (5): 210-29.
- Zhang QW, Gen LL, Cai WY, Techniques for extraction and isolation of natural products: a comprehensive review, Chinese Medicine, 2018; 13(20): 1-26.
- Chandra S, Khan S, Avula B, Lata H, Yang MH, ElSohly MA, et al., Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study, Evidence-based complementary and alternative medicine, 2014; 2014:253875
- Harborne A. Phytochemical methods a guide to modern techniques of plant analysis: springer science & business media; 1998.
- Sembiring EN, Elya B, Sauriasari R, Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb, Pharmacognosy journal, 2018; 10 (1): 123-127
- Gupta N, Mukerjee A, Mishra SB, Design, synthesis and molecular docking of vanillic acid derivatives as amylolytic enzyme inhibitors, Pharmaceutical Chemistry Journal, 2021; 55 (5): 427-35.
- Aljarah AK, Hameed IH, In vitro anti-diabetic properties of Methanolic extract of Thymus vulgaris using α-glucosidase and α-amylase inhibition assay and determination of its bioactive chemical compounds, Indian Journal of Public Health Research and Development, 2018; 9 (3): 388-92.
- Mechchate H, Es-Safi I, Louba A, Alqahtani AS, Nasr FA, Noman OM, et al., In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract, Molecules, 2021; 26 (2): 293.
- Narayan S, Mittal A, An oral acute toxicity study of extracts from Salvia splendens (scarlet sage) as per OECD guidelines 423, World Journal of Pharmaceutical Sciences, 2015, 3(3): 512-518.
- Assadi S, Shafiee SM, Erfani M, Akmali M, Antioxidative and antidiabetic effects of Capparis spinosa fruit extract on high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats, Biomedicine & Pharmacotherapy, 2021; 138: 111391.
- Bedi O, Krishan P, Investigations on acute oral toxicity studies of purpurin by application of OECD guideline 423 in rodents, Naunyn-Schmiedeberg's archives of pharmacology, 2020; 393: 565-71.
- 20. Poovitha S. and Parani M., In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.), 2015; 16(185): 1-15.
- Graziele Freitas de Bem G.F.b., Costa C.A., Santos I.B., Cordeiro V.d.S.C., Carvalho L.C.R.M.d. et al, Antidiabetic effect of Euterpe oleracea Mart. (açaí) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: A positive interaction, 2018; 13(6): 1-19.
- Singh R., Gholipourmalekabadi M., Shafikhani S.H., Animal models for type 1 and type 2 diabetes: advantages and limitations, 2024; 1-17.
- Furman BL., Streptozotocin-Induced Diabetic Models in Mice and Rats, 2021; 1-21.
- Ormazabal V., Nair S., Elfeky O., Aguayo C. et al, Association between insulin resistance and the development of cardiovascular disease, 2018; 17(122): 1-12.
- Shaikh IA , MM U, C K., Badiger S, et al, In Silico Molecular Docking and Simulation Studies of Protein HBx Involved in the Pathogenesis of Hepatitis B Virus-HBV, 2022; 27: 1-12.
- Forouhi NG, Wareham NJ, Epidemiology of diabetes, Medicine, 2019; 47 (1): 22-7.
- Adisakwattana S, Cinnamic acid and its derivatives: mechanisms for prevention and management of diabetes and its complications, Nutrients, 2017; 9 (2): 163.
- Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A., Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview, 2018; 5 (3): 1-16.
- Lu Y-h, Tian C-r, Gao C-y, Wang X-y, Yang X, Chen Y-x, et al., Phenolic profile, antioxidant and enzyme inhibitory activities of Ottelia acuminata, an endemic plant from south-western China, Industrial Crops and Products, 2019; 138: 111423.

 ETFLIN
Notification
ETFLIN
Notification






