Antioxidant Properties, α-Amylase and α-Glucosidase Inhibitory Activities of Maesobotrya barteri Leaves Extracts in Rats
by Godwin Ndarake Enin ★ , Basil Nse Ita, Paul Sunday Thomas, Jude Efiom Okokon, Blessing Ofonime Lawson, Chidera Getrude Ohanaka
Academic editor: Pilli Govindaiah
Sciences of Pharmacy 4(1): 20-31 (2025); https://doi.org/10.58920/sciphar0401294
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
10 Nov 2024
01 Mar 2025
04 Mar 2025
13 Mar 2025
Abstract: Maesobotrya barteri is widely used in Nigerian ethnomedicine to treat diabetes, arthritis, and infections. In this study, the methanol and aqueous leaf extracts' phytochemical constituents and antioxidant potentials were evaluated using standard procedures. At the same time, the enzyme inhibitory activity of methanol extract on α-amylase and α-glucosidase in rats was also investigated. The antioxidant properties of the extracts were evaluated by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and ferric reducing power (FRAP). Phytochemical screening of methanol and aqueous extracts revealed the presence of flavonoids, alkaloids, saponins, tannins, and cardiac glycosides. Total phenolics and flavonoids of the extracts ranged from 0.04 to 3.18 mg of GAE/g and from 27.70 to 57.70 mg of QE/g, respectively. Antioxidant analysis showed IC50 values of DPPH (192.95, 196.04, and 17.19 µg/mL) and FRAP (30.48, 37.64, and 38.15 µg/mL) for the methanolic extract, aqueous extract, and ascorbic acid, respectively. Assessment of the methanolic extract against α-amylase and α-glucosidase enzymes in rats at doses of 150, 300, and 450 mg/kg using starch, sucrose, and maltose as substrates, with acarbose as a reference drug, significantly reduced blood glucose levels (p < 0.05). These findings suggest that M. barteri leaf extract has antioxidant properties and inhibits both α-amylase and α-glucosidase enzymes in rats, likely due to the phytochemicals present in the extract.
Keywords: AntioxidantsAlpha-glucosidaseDiabetesPhytochemicalsMaesobotrya barteri
Introduction
Diabetes and its related complications are increasingly recognized as significant health challenges linked to oxidative stress and inflammation in humans. The rising prevalence of insulin resistance and type 2 diabetes has become a global health crisis. In 2015, there were an estimated 415 million cases of diabetes (1, 2). By 2019, it was revealed that over 463 million people worldwide were affected by diabetes, with 19.4 million cases in Africa, representing approximately 4.7% of the adult population aged 20 to 79 years. Projections suggested this number could rise to 783 million by 2045 (1-4). To manage diabetes, various treatments, including synthetic drugs like metformin and phenformin, have been widely prescribed. While these medications effectively enhance peripheral glucose uptake and reduce hepatic glucose production, long-term use can lead to undesirable side effects (5). In contrast, bioactive compounds in plants, fruits, and vegetables, which possess antidiabetic and antioxidant properties, have shown promise in alleviating diabetic symptoms and serve as valuable nutritional resources to combat oxidative stress.
Antioxidants are crucial in neutralizing free radicals and mitigating oxidative stress, which are linked to various diseases, including diabetes, cancer, cardiovascular issues, inflammation, thyroid disorders, and neurodegenerative conditions. Antioxidants help to prevent the formation of reactive oxygen species and capture harmful radicals while also repairing damaged nucleic acids, removing oxidized proteins, and restoring oxidized lipids with the aid of enzymes like hydrolases and phospholipases (6). Free radicals such as reactive oxygen, nitrogen, and chlorine species are the major causes of health problems globally (7). These highly reactive entities, including hydroxyl radicals, nitric oxide radicals, hydrogen peroxide, superoxide anions, lipid peroxides, and various singlet oxygen molecules, can harm nucleic acids, proteins, enzymes, and other vital biomolecules, ultimately disrupting their structure and function (8).
An imbalance between prooxidants and antioxidants in the body leads to oxidative stress. Endogenous antioxidants act as free radical scavengers and lipid peroxidation inhibitors, such as superoxide dismutase, glutathione, uric acid, melatonin, metal-binding proteins, and polyamines. However, they require support from exogenous antioxidants to effectively maintain the body’s functions (9, 10). Bioactive compounds, particularly those rich in polyphenols found in fruits, vegetables, and spices, are reliable sources of these antioxidants. Research has shown that diets high in vitamins, carotenoids, and phenolic compounds can protect human cells from prooxidant-related diseases (8, 11). To explore these benefits, various plant species have been screened for their antioxidant activities to expand natural antioxidants' ability to treat health conditions.
Maesobotrya barteri, a shrub from the Euphorbiacaea family, is native to several African regions, including the rainforest areas of Sierra Leone, Southern Nigeria, and Western Cameroon. It bears succulent white berries, commonly referred to as "squirrel cherry" in English and "Nyanyated" by the Ibibio people of Akwa Ibom State, Nigeria. However, some reports mention a black-purple variety. Ethnopharmacologically, the plant has been used to treat diabetes, malaria, dysentery, arthritis, mumps, and rheumatism (12, 13). Its twigs are used as chewing sticks, roots are infused in gin for arthritis treatment, and stems are employed for fencing and supporting yam tendrils (12, 13). A recent study has reported the presence of alkaloids, flavonoids, terpenoids, saponins, and tannins in the leaves of M. barteri (14)
Despite the fact that M. barteri is used traditionally in the southern part of Nigeria for the treatment of diabetes, malaria, dysentery, arthritis, mumps, and other ailments, there is scanty literature information on the antidiabetic activity of this plant. Inhibition of α-amylase and α-glucosidase study is one of the modes of antidiabetic activity. This study was therefore designed to evaluate the antidiabetic and antioxidant activities of M. barteri leaf extracts by analyzing their phytochemical components, DPPH, and FRAP, as well as evaluating the effects of the methanolic extract on alpha-amylase and alpha-glucosidase activities in rats. The findings of this research will contribute to the scientific understanding of the plant's antidiabetic potential and its phytochemical composition.
Methodology or Experimental Section
Materials
The materials used include M. barteri leaf extract, stirrer, beakers, 1 mL syringe, iodine-potassium iodide, aluminum chloride, sodium hydroxide, hydrochloric acid, ferric chloride, sodium carbonate, sulphuric acid, phosphate buffer, and potassium ferricyanide, oral gastric gavage, weighing balance, gloves, scissors, glucometer and strips (fine test), distilled water, acarbose, starch, sucrose, maltose (from Sigma Aldrich, USA).
Plant Materials
Plant Collection, Identification, and Preparation
M. barteri leaves were harvested from a forest in Ediene Attai Village in Oruk Anam Local Government Area of Akwa Ibom State, Nigeria, in March 2024. The plant identification was carried out at the Department of Botany and Ecological Study, Faculty of Biological Sciences, University of Uyo, Nigeria. The fresh leaves were washed with flowing water, air-dried at ambient temperature for two weeks, and reduced into a fine powder using a laboratory mill.
Plant Extraction
The method of Ouandaogo et al. (2023) was used to extract the plant sample (15). Ninety grams (90 g) of the finely powdered leaves were placed in conical flasks and extracted with 70% methanol. The flasks were placed on a flat plate mechanical shaker (model: platform ZD 881) and macerated for 14 hours at 25 °C. The resulting solution was filtered, and the filtrate was concentrated to dryness in vacuo to obtain the methanol extract. Another 90 g of the powdered leaves were macerated with distilled water at 65 °C according to the previous method to obtain the aqueous extract (13). The methanol and aqueous extracts were weighed, the percentage yield calculated as shown below, and the extracts were refrigerated until use.
Qualitative Phytochemical Screening
Preliminary Phytochemical Screening
Phytochemical test for flavonoids, alkaloids, saponins, tannins, cardiac glycosides, and anthraquinones were conducted according to standard methodologies (13).
Flavonoid Test: The extract (0.2 g) was gently warmed in ethanol and filtered. Two pieces of magnesium chips were then added, followed by 2 mL of concentrated HCl. No observable color change indicated the absence of flavonoids.
Sodium Hydroxide Test: The extract (0.2 g) was dissolved in 2 mL of distilled water and filtered. Then, 1 mL of 5% sodium hydroxide solution was slowly added. For both extracts, the solution changed from dark green to yellow, indicating the presence of flavonoids.
Saponin Test: The extract (0.2 g) was placed in a test tube with 5 mL of distilled water, shaken vigorously for 60 seconds, and left to stand for 15 minutes. The presence of persistent frothing on the surface confirmed the presence of saponins.
Alkaloid Test: The extract (0.2 g) was dissolved in ethanol, followed by the addition of 5 mL of 1% diluted HCl. The mixture was warmed and filtered. Then, 2 mL of the filtrate was placed in a test tube, and Dragendorff’s reagent was added. The appearance of a reddish color indicated the presence of alkaloids.
Tannin Test: The extract (0.2 g) was mixed with 5 mL of distilled water, stirred, and filtered. To 2 mL of the filtrate, two drops of 1% ferric chloride solution were added and shaken. The formation of a black-colored solution confirmed the presence of tannins.
Cardiac Glycoside Test: The extract (0.2 g) was dissolved in chloroform, and 1 mL of concentrated sulfuric acid (H₂SO₄) was carefully added down the side of the test tube. The presence of a reddish-brown color at the interface indicated the presence of a steroidal ring, characteristic of the aglycone portion of cardiac glycosides.
Anthraquinone Test: The extract (0.2 g) was mixed with distilled water, shaken with 3 mL of benzene, and filtered. To the filtrate, 5 mL of 10% ammonia solution was added, and the mixture was shaken for 60 seconds. No significant color change was observed, indicating the absence of anthraquinones.
Quantitative Phytochemical Screening
Total Phenolic Content
The total phenolic content (TPC) was determined spectrophotometrically using a modified version of the method by Kim et al. (2003) (16). A 0.5 mL sample (1 mg/mL in methanol) was mixed with 2.5 mL of 10% Folin-Ciocalteu reagent and 2 mL of 7% Na₂CO₃. The mixture was vortexed for 15 seconds and incubated in the dark at 40 °C for 30 minutes to allow color development. Absorbance was measured at 765 nm using a UV-Vis spectrophotometer (Model: Techmel and Techmel, USA). A calibration curve was generated using gallic acid solutions (10–100 μg/mL), and TPC was calculated by extrapolating sample absorbance values from the standard curve. Results were expressed as mg of gallic acid equivalent per gram of dry weight.
Total Flavonoids Content
The total flavonoid composition was obtained utilizing the protocol of Subhashini et al. (2010). A 1 mg/mL extract solution was diluted with 200 µL of distilled water, followed by the addition of 150 µL of 5% sodium nitrite (NaNO₂) solution. This mixture was incubated for 5 minutes and added to 150 µL of 10% AlCl3.6H2O. After 6 minutes, 2 mL of 1M NaOH was added. The absorbance reading was noted using a UV-Vis spectrophotometer at 510 nm and the total flavonoid content was expressed as mg of quercetin (QE) equivalent per gram dry weight (17).
Radical Scavenging Activity Assay
The antioxidant activity of the extract was assessed using the DPPH radical scavenging assay, following a standard methodology (18). A 1 mL aliquot of 0.1 mM DPPH solution was mixed with 3 mL of the test solution containing the extract and ascorbic acid. The mixture was stirred for one minute and then incubated in the dark for 30 minutes. Absorbance was measured at 517 nm using a UV-Vis spectrophotometer (Techmel and Techmel, USA).
Ferric Reducing Antioxidant Power Assay
The ferric reducing power was determined according to the method of Ali et al. (2020). Various concentrations (µg/mL) of the extract were added to (1 mL) of 200 mM sodium phosphate buffer (pH 6.6) and 1 mL of (0.69 mL) potassium ferricyanide, [K3[Fe(CN)6]. The mixture was incubated at 50 °C for 20 minutes. Thereafter, trichloroacetic acid (1 mL, 10%) was prepared and dissolved with 50 mL of distilled water. The mixture was centrifuged at 650 rpm for 10 minutes. The upper layer (4 mL) was mixed with (4 mL) of deionised water and 0.8 mL of 0.1% of ferric chloride anhydrous (FeCl3), and the absorbance was measured with UV-Vis spectrophotometer at 700 nm. The same procedure was repeated with various concentrations of ascorbic acid. Higher absorbance indicates higher reducing power (19).
Experimental Animals
Albino Wistar rats (120 -135 g) of either sex maintained at the animal house of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Uyo, Nigeria, were used for the study. The animals were housed in standard cages and were maintained on standard pelleted feed (Guinea feed) and water ad libitum. The approval for animal studies was obtained from the College of Health Sciences Animal Ethics Committee, University of Uyo (UU/CS/AE/14/63).
Alpha-Amylase Inhibitory Study Using Starch as Substrate
Thirty Wistar rats were randomly divided into six groups, with five rats per group. All rats were fasted for 18 hours before the experiment, and their fasting blood glucose levels were measured at 0 minutes (before administration).
- Group I (Normal Control): Received distilled water (10 mL/kg).
- Group II: Administered starch (2 g/kg) with distilled water (10 mL/kg) as a vehicle.
- Group III: Administered starch (2 g/kg) along with the standard drug acarbose (100 mg/kg).
- Groups IV, V, and VI: Administered starch (2 g/kg) along with M. barteri leaf extract at doses of 150, 300, and 450 mg/kg, respectively.
All substances were administered orally. Blood glucose levels were monitored at 60, 90, 120, and 180 minutes to assess the effect of the extract on enzyme activity.
Sucrose Inhibitory Study (Using Sucrose as Substrate)
Thirty Wistar rats were randomly divided into six groups, with five rats in each group. All rats were fasted for 18 hours before the experiment, and their fasting blood glucose levels were measured at 0 minutes (before administration).
- Group I (Normal Control): Received distilled water (10 mL/kg).
- Group II: Administered sucrose (2 g/kg) with distilled water (10 mL/kg) as a vehicle.
- Group III: Administered sucrose (2 g/kg) along with the standard drug acarbose (100 mg/kg).
- Groups IV, V, and VI: Administered sucrose (2 g/kg) along with M. barteri leaf extract at doses of 150, 300, and 450 mg/kg, respectively.
All substances were administered orally. Blood glucose levels were monitored at 60, 90, 120, and 180 minutes to evaluate the effect of the extract on enzyme activity.
Maltose Inhibitory Study (Using Maltose as Substrate)
Thirty Wistar rats were randomly divided into six groups, with five rats in each group. All rats were fasted for 18 hours before the experiment, and their fasting blood glucose levels were measured at 0 minutes (before administration).
- Group I (Normal Control): Received distilled water (10 mL/kg).
- Group II: Administered maltose (2 g/kg) with distilled water (10 mL/kg) as a vehicle.
- Group III: Administered maltose (2 g/kg) along with the standard drug acarbose (100 mg/kg).
- Groups IV, V, and VI: Administered maltose (2 g/kg) along with M. barteri leaf extract at doses of 150, 300, and 450 mg/kg.
All administrations were performed orally. Blood glucose levels were monitored at 60, 90, 120, and 180 minutes to evaluate the effect of the extract on enzyme activity.
Blood Glucose Determination
Blood glucose concentration was measured using a glucometer (Fine Test) according to the manufacturer's specifications. Blood samples were obtained by pricking the tip of each rat’s tail, and a drop of blood was applied to a test strip.
The glucometer operates using an electrochemical detection system based on the glucose oxidase method. Each disposable reagent strip contains an electrode impregnated with glucose oxidase, which reacts with glucose in the blood sample upon contact with the membrane covering the reagent pad. This reaction produces gluconic acid and generates an electric current. An electrochemical mediator transfers electrons to the electrode surface, and the sensor measures the resulting current. The magnitude of the generated current is proportional to the glucose concentration in the blood sample, providing an accurate measurement of blood glucose levels (21).
Statistical Analysis
Data obtained from this work were analysed statistically using one –way ANOVA followed by Tukey-Kramer multiple comparison test using Instat Graphpad software, (San Diego, USA). Differences between means were considered significant at p < 0.05 and very significant at p < 0.001.
Results and Discussion
Plant Extraction
Extraction of M. barteri leaves with methanol afforded methanol crude extract (9.5% yield). However, aqueous extraction of the leaves gave 14% yield of the aqueous extract. The results showed that aqueous extraction afforded the highest yield showcasing the effect of solvent polarity (see Table 1).
Table 1. Yield of methanol and aqueous extracts.
Extract | Sample (g) | Extract (g) | Yield (%) |
Methanol | 90 | 8.6 | 9.5 |
Aqueous | 90 | 12.3 | 14 |
Preliminary Phytochemical Screening
The results of the preliminary phytochemical screening of M. barteri leaf extracts showed the presence of alkaloid, flavonoid, saponins, tannins and cardiac glycosides in the methanol and aqueous extracts while the presence of anthraquinones were not detected in both extracts during the preliminary phytochemical analysis (see Table 2).
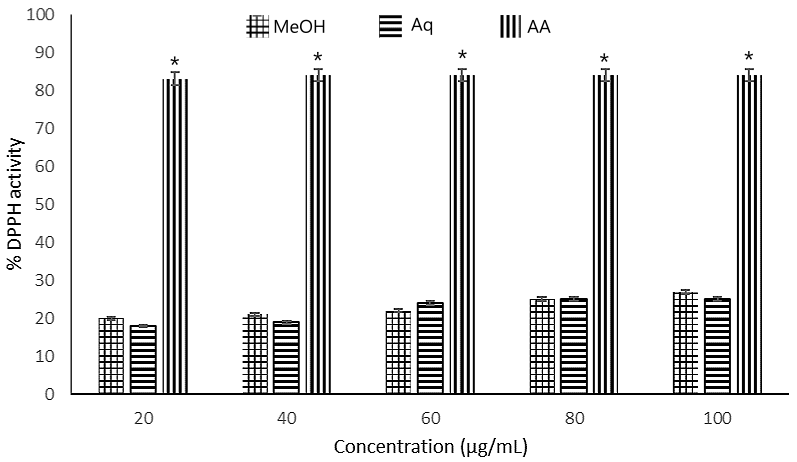
Figure 1. DPPH radical scavenging activity of different concentrations (20-100 µg/mL) of Maesobotrya barteri leaves methanol and aqueous extracts. Note: MeOH = methanol extract; Aq = aqueous extract; AA = ascorbic acid; and DPPH = 1,1-diphenyl-2-picryl-hydrazyl free radical. (*, p < 0.05) indicates a significant difference compared to other groups.
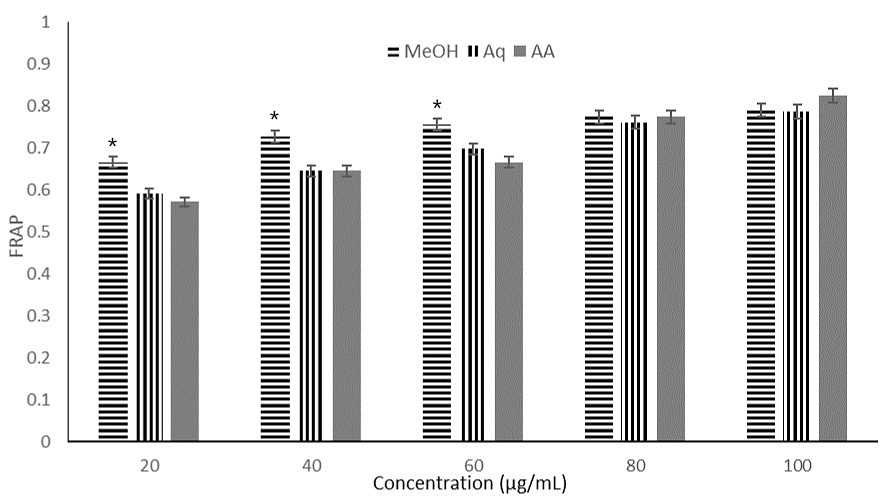
Figure 2. Ferric reducing antioxidant power (FRAP) activity of different concentrations (20-100 µg/mL) of Maesobotrya barteri leaves methanol and aqueous extracts. Note: MeOH = methanol extract; Aq = aqueous extract; AA = ascorbic acid; and DPPH = 1,1-diphenyl-2-picryl-hydrazyl free radical. (*, p < 0.05) indicates a significant difference compared to other groups.
Table 2. Preliminary phytochemical screening of methanol and aqueous extracts.
Phytochemicals | Results | |
Methanol | Aqueous | |
Alkaloids | + | + |
Flavonoids | + | + |
Saponins | + | |
Tannins | + | + |
Anthraquinones | – | – |
Cardiac Glycosides | + | + |
Note : Key: + = Present; – = absent. | ||
Total Phenolic and Flavonoid Contents
The total phenolic content in the extracts ranged from 0.04 mg GAE/g to 3.18 mg GAE/g. From the gallic acid calibration curve expression (y = 0.014x + 0.1625), the aqueous extract of M. barteri showed the highest phenolic content (3.18 mg GAE/g) compared to the methanol extract (0.04 mg GAE/g). The reverse was observed for the total flavonoid content of the extracts. The aqueous extract had the least flavonoid content (27.70 mg QE/g) while the methanolic extract revealed the highest flavonoid content (57.70 mg QE/g), representing about a 2-fold increase in the total flavonoid content (see Table 3).
Table 3. Total phenolics, flavonoids, DPPH values, and FRAP values of methanol and aqueous extracts of Maesobotrya barteri leaves.
Assay | Methanol | Aqueous | Ascorbic acid |
TPC (mg GAE/g) | 0.04 | 3.18 |
|
TFC (mg QE/g) | 57.70 | 27.70 |
|
DPPH IC50 (µg/mL) | 192.95 | 196.04 | 17.19 |
FRAP IC50 (µg/mL) | 30.48 | 37.64 | 38.15 |
Radical Scavenging and Ferric Reducing Antioxidant Power Assay
Both extracts scavenged radical of DPPH and exhibited reducing potential in a concentration-dependent manner (see Figure 1 and 2). The methanolic extract scavenged radicals of DPPH with higher inhibition percentage compared to the aqueous extract even though the effect was not so remarkable. At 100 µg/mL concentration, methanol extract scavenged 27% of DPPH radicals while the aqueous extract was able to scavenged 25% of DPPH radicals. The standard drug (ascorbic acid) exhibited 84% inhibitory activity. At 40 µg/mL, the aqueous extract scavenged 21% of the DPPH radicals comparable to the 19% scavenging activities of the methanol extract. For DPPH, the IC50 values of the methanol and aqueous extracts were 192.50 µg/mL and 196.04 µg/mL respectively whereas ascorbic acid exhibited IC50 value of 17.19 µg/mL. For FRAP, again methanol extract exhibited the highest reducing power (IC50 = 30.48 µg/mL, aqueous extract demonstrated the least FRAP activity (IC50 = 37.64 µg/mL). Ascorbic acid, the standard drug showed IC50 value of 38.15 µg/mL, indicating that methanol extract exhibited the highest reducing power. The antioxidant activity of extracts also showed strong correlation with the total flavonoids and phenolics in the extracts (see Table 4).
Table 4. Correlation between antioxidant assays, total phenolics and total flavonoid.
| DPPH | FRAP | TP | TF |
DPPH | 1 |
|
|
|
FRAP | 0.99 | 1 |
|
|
TP | 0.79 | 0.96 | 1 |
|
TF | 0.79 | 0.95 | 0.99 | 1 |
Administration of starch (2 g/kg) to fasted rats caused varying percentages of increase in blood glucose levels of the treated animals after one hour. The percentages were starch (40.31%), M. barteri leaf extract-treated groups (11.73 - 16.78%), and acarbose-treated group (11.06%). These increases were reduced after 120 minutes with the group treated with the low and middle doses of the extract (500 and 1000 mg/kg) having percentage increases of 1.44 and 7.68 % respectively. All the extract-treated groups had their BGL reduced to a normal level at 180 minutes and this was sustained throughout the study. Also, co-administration of the starch with acarbose prominently inhibited the rise in the blood glucose concentrations (see Table 5).
Administration of maltose (2 g/kg) to fasted rats caused varying percentages of increase in blood glucose levels of the treated animals after one hour. The percentages were maltose (44.95%), M. barteri leaf extract-treated groups (1.29 - 41.39%), and acarbose-treated group (0.77%). These increases were reduced to normal after 120 minutes and this was sustained throughout the duration of the study. Also, co-administration of the maltose with acarbose prominently inhibited the rise in the blood glucose concentrations (see Table 6).
Table 5. Effect of methanol leaf extract of Maesobotyra barteri on blood glucose level of rat after oral administration of starch load.
Treatment | Dose (mg/kg) | Blood glucose level (mg/dL, [%]) in minutes | |||
0 min | 60 min | 120 min | 180 min | ||
Control (normal saline) | - | 86.00 ± 11.53 | 87.66 ± 7.62 [1.93] | 91.0 ± 7.50 [5.81] | 80.00 ± 6.02 |
Starch (negative control) | 2000 | 80.0 ± 4.54 | 112.25 ± 4.73 [40.31] | 92.50 ± 1.70 [15.62] | 87.25 ± 6.52 [9.06] |
Acarbose (positive control) | 100 | 72.33 ± 2.69 | 80.33 ± 7.21 [11.06] | 74.0 ± 1.00 [2.30] | 72.33 ± 8.68 |
Extract | 150 | 95.33 ± 4.48 | 111.33 ± 11.31 [16.78] | 102.66 ± 2.33a [7.68] | 90.0 ± 4.00a |
300 | 92.66 ± 0.33 | 104.0 ± 11.54 [11.73] | 94.0 ± 6.24b [1.44] | 80.0 ± 3.00a | |
450 | 89.33 ± 2.02 | 102.66 ± 8.09 [14.92] | 105.0 ± 6.65a [17.54] | 88.66 ± 6.76 | |
Note: Data is expressed as MEAN ± SEM, significant at ap < 0.05 and bp < 0.01, when compared to control (n=6). Values in brackets are percentage increases in blood glucose concentrations compared to 0 min in the same group. | |||||
Table 6. Effect of methanol leaf extract of Maesobotyra barteri on blood glucose level of rat after oral administration of maltose load.
Dose (mg/kg) | Blood glucose level (mg/dL, [%]) in minutes | ||||
0 min | 60 min | 120 min | 180 min | ||
Normal control | - | 86.00 ± 11.53 | 87.66 ± 7.62 [1.93] | 91.0 ± 7.50 [5.81] | 80.00 ± 6.02 |
Maltose (negative control) |
| 86.75 ± 2.52 | 125.75 ± 1.65 [44.95] | 99.50 ± 2.90 [12.75] | 88.0 ± 1.68 [1.44] |
Acarbose (positive control) | 100 | 85.34 ± 1.36 | 86.0 ± 2.20 [0.77] | 84.26 ± 1.14a | 82.28 ± 2.26 |
Extract | 150 | 102.33 ± 4.70 | 103.66 ± 3.28 [1.29] | 87.33 ± 1.66 | 78.0 ± 4.93 |
300 | 94.66 ± 5.36 | 122.33 ± 7.53 [29.23] | 82.66 ± 2.60 | 79.33 ± 3.18 | |
450 | 100.66 ± 7.53 | 142.33 ± 7.31 [41.39] | 92.0 ± 7.23 | 88.0 ± 14.84 | |
Note: Data is expressed as MEAN ± SEM, significant at ap < 0.05, bp < 0.01, when compared to control (n=6). Values in brackets are percentage increases in blood glucose concentrations compared to 0 min in the same group. | |||||
Table 7. Effect of methanol leaf extract of Maesobotyra barteri on blood glucose level of rat after oral administration of sucrose load.
Treatment | Dose (mg/kg) | Blood glucose level (mg/dL, [%]) in minutes | |||
0 min | 60 min | 120 min | 180 min | ||
Normal control | - | 86.00 ± 11.53 | 87.66 ± 7.62 [1.93] | 91.0 ± 7.50 [5.81] | 80.00 ± 6.02 |
Sucrose (negative control) |
| 81.0 ± 4.50 | 112.66 ± 1.49a [39.08] | 97.33 ± 1.63 [20.16] | 94.15 ± 4.81 [16.23] |
Acarbose (positive control) | 100 | 90.33 ± 2.48 | 82.0 ± 6.00 | 71.66 ± 3.75 | 78.0 ± 3.78 |
Extract | 150 | 93.33 ± 2.18 | 92.33 ± 3.52 | 85.33 ± 3.18 | 76.0 ± 2.08 |
300 | 84.66 ± 1.84 | 97.33 ± 0.33 [14.96] | 89.33 ± 1.20 [5.51] | 67.66 ± 3.71 | |
450 | 90.66 ± 7.83 | 101.66 ± 5.17 [12.13] | 85.33 ± 4.17 | 81.66 ± 3.81 | |
Note: Data is expressed as MEAN ± SEM, significant at ap < 0.05, bp < 0.01, when compared to control (n = 6). Values in bracket are percentage increases in blood glucose concentrations compared to 0 min in the same group. | |||||
Administration of sucrose (2 g/kg) to fasted rats caused varying percentages of increase in blood glucose levels of the treated animals after one hour. The percentages were sucrose (39.08%), and M. barteri leaf extract-treated groups (0 - 14.96%). The low dose (500 mg/kg) and acarbose-treated groups had no increment in BGL. These increases were reduced to normal after 120 minutes with only the group treated with the middle dose (1000 mg/kg) of the leaf extract having BGL increment of 5.51 %. All the groups treated with the leaf extract had their BGL reduced to a normal level at 180 minutes and this was sustained throughout the study (see Table 7).
Plants are recognized for producing a variety of chemical compounds which have numerous commercial and industrial uses, including flavours, aromas, fragrances, enzymes, preservatives, cosmetics, natural pigments, and bioactive compounds. Research into these phytochemicals has led to the isolation and identification of many natural products, such as phenolics, flavonoids, organic acids, steroids, terpenes, lignans, glycosides, and alkaloids (2). Among these, flavonoids, phenolic acids, and tocopherols are particularly valued for their potent antioxidant properties, which help scavenge free radicals in the human body (22). This study examined the in vitro antioxidant activity of methanol and aqueous extracts from M. barteri leaves, as well as their effects on blood glucose levels. We assessed the extracts' ability to scavenge the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, their ferric reducing antioxidant power, total phenolic and flavonoid content, and their inhibitory effects on the alpha-amylase and alpha-glucosidase in Wistar rats.
In terms of total phenolic content, the aqueous extract had higher content (7.68 mg GAE/g) compared to the methanol extract, while total flavonoid content was higher in the methanolic extract (271.40 mg QE/g), suggesting that extraction with methanol afforded a more potent path to flavonoids. In the DPPH scavenging assay, both extracts demonstrated radical scavenging activity against DPPH radicals, the methanolic extract showed a slightly higher inhibition percentage than the aqueous extract although the difference was minimal. At 60 µg/mL, the aqueous extract scavenged 24% of DPPH radicals, while the methanolic extract scavenged 22%. At 100 µg/mL, the methanol extract scavenged 27% of DPPH radicals, compared to 25% by the aqueous extract. The standard drug, ascorbic acid, demonstrated a much higher inhibition (84%), as shown in Figure 2. The IC50 values, derived from plotting concentration (µg/mL) against percentage inhibition for DPPH, were: methanol extract (192.95 µg/mL), aqueous extract (196.04 µg/mL), and ascorbic acid (17.19 µg/mL), as listed in Table 2. These results indicate that both the methanolic and aqueous extracts of Maesobotrya leaves showed weak DPPH radical scavenging activity compared to the control (ascorbic acid), although the methanol extract exhibited a better scavenging radical activity. The high IC50 values (>100 µg/mL) suggest that these extracts do not possess strong antioxidant activity. The ferric reducing antioxidant power was evaluated by plotting mean absorbance values against extract concentrations (µg/mL) in the range of 20-100 µg/mL (see Figure 3). At 40 µg/mL, the absorbance values were 0.727, 0.645, and 0.571 for methanol, aqueous, and ascorbic acid, respectively. At 100 µg/mL, the absorbance values increased to 0.790, 0.786, and 0.824 for methanol, aqueous, and ascorbic acid, respectively. The IC50 values were: methanol extract (30.48 µg/mL), aqueous extract (37.64 µg/mL), and ascorbic acid (38.15 µg/mL), as shown in Figure 2. In terms of the reducing power the results demonstrate that the methanol extract had a higher reducing power than the aqueous extract. Ferric reducing assay has been described as one of the useful tools in profiling antioxidant capabilities (4, 34). The relatively low phenolic and flavonoid content in the methanol extract (0.04 mg GA/g and 57.70 mg QE/g) and the aqueous extract (3.18 mg GA/g and 27.70 mg QE/g) may explain the observed weak antioxidant activity, suggesting a correlation between the plant's antioxidant properties and its polyphenolic content. Total phenolics showed good correlation with DPPH activity (r2 = 0.79) and reducing power (r2 = 0.96) respectively. Similar correlation was observed for the flavonoids demonstrating the correlation between antioxidant properties and polyphenolic compounds (see Table 4).
The methanol extract significantly inhibited increases in blood glucose concentrations following starch administration, independent of the dose. Starch is a vital carbohydrate source for humans and other animals, and its digestion primarily involves two enzymes: α-amylase and α-glucosidase. α-amylase, found in saliva and pancreatic juices, breaks down starch by cleaving α-(1→4)-D-glycosidic bonds, producing smaller oligosaccharides and disaccharides (23). These disaccharides are further reduced to monosaccharides by membrane-bound α-glucosidase enzymes (24). Inhibition of these enzymes slows carbohydrate digestion, resulting in a more gradual increase in blood glucose levels, as observed in our study.
Research indicates that reducing postprandial hyperglycemia can effectively manage early-stage of diabetes by inhibiting α-glucosidase and α-amylase, which slows glucose absorption and mitigates plasma glucose spikes after meals (25-27). Acarbose, voglibose, and miglitol are commercial antidiabetic medications however, these medications cause side effects such as flatulence, stomach discomfort, and allergic reactions (28). The inhibitory effects of plant extracts on alpha amylase and alpha glucosidase enzymes activities have been documented (29, 30) and has been linked to the bioactive constituents in the extracts (31, 32). Many works have shown that various plant bioactive compounds possess hypoglycemic properties, affecting multiple targets, proteins, and enzymes (33-35). Alkaloids, flavonoids, anthocyanins, terpenoids, phenolic compounds, and glycosides have been identified as bioactive α-glucosidase inhibitors with significant impacts on diabetes management. These compounds influence glucose transportation in the body and intestinal glycosidase activity (36-38). Additionally, polyphenols act as metal ion chelators and protein precipitation agents (37, 38).
Notably, when co-administered with maltose and sucrose, the methanol extract of M. barteri significantly inhibited blood glucose increases, albeit lower compared to acarbose. Phytochemical screening revealed that both extracts contained flavonoids, alkaloids, saponins, tannins, and glycosides. Tannins, flavonoids, terpenoids, and phenolic acids are known for their strong antioxidant, antidiabetic, and anti-inflammatory properties (8, 39). Flavonoids exist in various forms, including flavones, isoflavones, flavanones, and flavanols and are reported to inhibit α-glucosidase. For instance, as shown in Figure 3, Calodenin A and (-)-epigallocatechin-gallate (compound 1 & 2) exhibit significant α-glucosidase inhibition (IC50: 0.4 µM and IC50: 5.2 µM, respectively) compared to the control, acarbose (IC50: 93.6 µM and IC50: 1400 µM) respectively, (40, 41). Similarly, procyanidin A2 (compound 3) demonstrated efficacy in managing postprandial diabetes with an IC50 of 0.27 g/mL (42). Phenolic acids such as caffeic acid and vanillic acid (compound 4 & 5) also showed superior α-glucosidase inhibition compared to standard drugs, acarbose (IC50: 0.39 and IC50: 8.38, respectively) (42). Furthermore, a study by Chen et al. (2020) reported that a diterpenoid, taxumariene F (compound 6) showed better inhibitory activity, with an IC50 of 3.7±0.75 µM when compared to the standard antidiabetic drug, acarbose (IC50 of 155.86±4.12 µM). Their findings also indicated that the activity of the diterpenoid was due to the presence of the tricycle, epoxy and acetoxy groups in the compound (43). While the inhibition of alpha amylase and alpha glucosidase study helps to understand the mechanism of antidiabetic activity, the antioxidant potential of a plant extract could enhance its antidiabetic effects by lowering oxidative stress, boosting insulin sensitivity, reducing inflammation, and safeguarding beta-cells from destruction by free radicals generated in the body in diabetic condition (31-36; 44). These all play a crucial role in regulating blood sugar levels and preventing complications of diabetes.
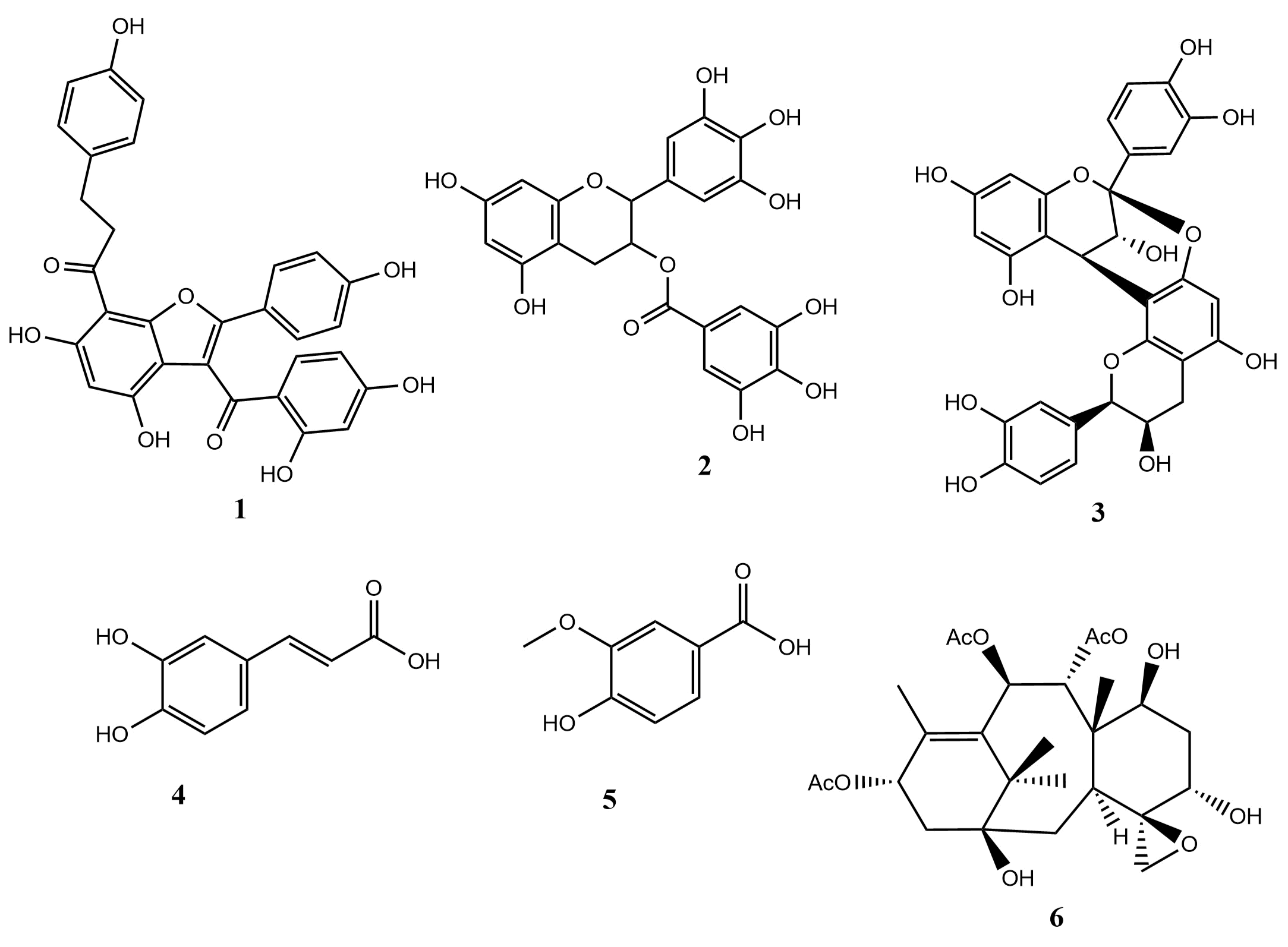
Figure 3. 2D chemical structure of alodenin A (compound 1), (-)- epigallocatechin-gallate (compound 2), procyanidin A2 (compound 3), caffeic acid (compound 4), vanillic acid (compound 5), and taxumariene (compound 6).
Flavonoids and other polyphenols are recognized for their antioxidant properties and role in managing postprandial hyperglycemia, while tannins significantly influence carbohydrate metabolism. Terpenoids contribute to plant growth, development, and stress resilience (28, 45). Given the increasing prevalence of diabetes, investigating natural products is a promising avenue for developing new hypoglycemic treatments. Future research should aim to isolate and characterize these bioactive compounds, examining the understanding of their molecular mechanisms to facilitate the design of more effective antidiabetic derivatives. Additionally, exploring the synergistic effects of these compounds in conjunction with existing antidiabetic therapies may lead to innovative strategies for managing postprandial diabetes.
Conclusion
The findings of this study suggested that the leaves extracts of M. barteri possess phytochemicals such as phenols, flavonoids and tannins which are known free radical scavengers. Their ability to scavenge free radicals in the antioxidant analysis could be implicated in the antidiabetic study in which the methanol extract showed inhibition of α-amylase and α-glucosidase enzymes being one of the modes of antidiabetic activity.
Declarations
Acknowledgment
The authors are grateful to the University of Uyo for the provision of Laboratory space.
Ethics Statement
The approval for animal studies was obtained from the College of Health Sciences Animal Ethics Committee, University of Uyo (UU/CS/AE/14/63). Ethical conditions guidelines for the care and use of Laboratory Animals, governing the conduct of experiments with life animals, were strictly maintained.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- International Diabetes Federation. IDF Diabetes Atlas. 7th Edition. International Diabetes Federation, Brussels. 2015;145p.
- Vikram A, Tripathi DN, Kumar A, Singh S. Oxidative stress and inflammation in diabetic complications. International Journal of Endocrinology. 2014; 679754. https://doi.org/10.1155/2014/679754.
- International Diabetes Federation. IDF Diabetes Atlas. 10th edition. Brussels, Belgium: International Diabetes Federation. 2021.
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. On behalf of the IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019; 57:107843.
- Deshmukh CD, Jain A, Nahata B. Diabetes mellitus: a review. Int. J. Pure Appl. Biosci. 2015; 3(3):224-230. www.ijpab.com.
- Zujko ME, Witkowska AM. Dietary antioxidants and chronic diseases. Antioxidants, 2023; 12(2):362. https://doi.org/10.3390/antiox12020362.
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry. 2015; 97:55-74. https://doi.org/10.1016/j.ejmech.2015.04.040.
- Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future Journal of Pharmaceutical Sciences, 2021; 7(25):1-13. https://doi.org/10.1186/s43094-020-00161-8.
- Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: A review. European Journal of Medicinal Chemistry. 2019; 178: 687-704. https://doi.org/10.1016/j.ejmech.2019.06.010.
- Muscolo A, Mariateresa O, Giulio T, Mariateresa R. Oxidative stress: the role of antioxidant phytochemicals in the prevention and treatment of diseases. International Journal of Molecular Sciences. 2024; 25(6):3264. https://doi.org/10.3390/ijms25063264.
- Wanjala WK, Kiambi MJ, Piero NM. Phytochemical Screening and In Vitro Evaluation of the Antioxidant Potential of Dichloromethane Extracts of Strychnos henningsii Gilg. and Ficus sycomorus L. The Scientific World Journal. 2023; (1):8494176. https://doi.org/10.1155/2023/8494176.
- Etukudo I. Ethnobotany. Conventional and Traditional Uses of Plants. Vol. 1. The Verdict Press, Uyo, Nigeria. 2003; 191
- Ubulom PM, Ettebong EO, Akpabio EI, Etokakpan KE. Evaluation of antiplasmodial activity of ethanol extract and fractions of Maesobotrya barteri root. Journal of Pharmacy & Bioresources. 2017; 14(1):68-74. https://dx.doi.org/10.4314/jpb.v14i1.9.
- Ajuru, MG, Wilson V. Phytochemical and Proximate Analysis of Leaves and Stem of an under Exploited Medicinal Plant, Maesobotrya barteri (Baill) Hutch. International Research Journal of Biological Sciences. 2014; 13(3):1-8.
- Ouandaogo HS, Diallo S, Odari E, Kinyua J. Phytochemical Screening and GC-MS Analysis of Methanolic and Aqueous Extracts of Ocimum kilimandscharicum leaves. ACS omega. 2023; 8(50):47560-47572. htps://doi.org/10.1021/acsomega.3c05554.
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry. 2003; 81(3):321-326. https://doi.org/10.1016/S0308-8146(02)00423-5.
- Subhashini R, Rao UM, Sumathi P, Gunalan G. A comparative phytochemical analysis of cocoa and green tea. Ind. J. Sci Technol. 2010; 3(2):188-192. http://www.indjist.org.
- Shekhar TC, Anju G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. American Journal of Ethnomedicine. 2014; 1(4): 244-249. http://www.ajethno.com.
- Ali BM, Boothapandi M, Nasar AS. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data in Brief. 2020; 28:104972. https://doi.org/10.1016/j.dib.2019.104972.
- Gidado A, Watafua M, Sa'ad RS, Tagi HS, Abdullahi S. Alpha-amylase, maltase and sucrase inhibitions by aqueous leaf extracts of Anacardium occidentale (Anacardiacea) and Piliostigma reticulatum (Caesalpiniaceae) in rats. Tropical Journal of Natural Product Research. 2019; 3(6):210-215. https://doi.org/10.26538/tjnpr/v3i6.5.
- World Health Organization. Glucose analyser. Core medical equipment information. www.who.int/medical_devices/en/index.html. 2011; Accessed 5 June, 2019.
- de Melo EB, da Silveira GA, Carvalho I. 2006. α-and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron. 2006; 62(44):10277-10302. https://doi.org/10.1016/j.tet.2006.08.055.
- Yang Y, Jia X, Xie H, Wei X. Dihydrochalcone C-glycosides from Averrhoa carambola leaves. Phytochemistry. 2020; 174:112364. https://doi.org/10.1016/j.phytochem.2020.112364.
- Kalra S. Alpha Glucosidase Inhibitors. The Journal of Pakistan Medical Association. 2014; 64(4):474-476.
- Park CJ, Han JS. Hypoglycemic effect of jicama (Pachyrhizus erosus) extract on streptozotocin-induced diabetic mice. Preventive Nutrition and Food Science. 2015; 20(2):88.
- Hiyoshi T, Fujiwara M, Yao Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. Journal of Biomedical Research. 2017; 33(1):1
- Morais FS, Canuto KM, Ribeiro PR, Silva AB, Pessoa OD, Freitas CD, Alencar NM, Oliveira AC, Ramos MV. 2020. Chemical profiling of secondary metabolites from Himatanthus drasticus (Mart.) Plumel latex with inhibitory action against the enzymes α-amylase and α-glucosidase: In vitro and in silico assays. Journal of Ethnopharmacology. 2020; 253:112644.
- Shehadeh MB, Suaifan GA, Abu-Odeh AM. Plants secondary metabolites as blood glucose-lowering molecules. Molecules. 2021; 26(14):4333. https://doi.org/10.3390/molecules26144333.
- Proença C, Freitas M, Ribeiro D, Oliveira EF, Sousa J, Tome SM, et al. α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure–activity relationship study. Journal of Enzyme Inhibition and Medicinal Chemistry. 2017; 32(1):1216-1228. Https://doi.org/10.1080/14756366.2017.1368503.
- Yang CY, Yen YY, Hung KC, Hsu SW, Lan SJ, Lin HC. Inhibitory effects of pu-erh tea on alpha glucosidase and alpha amylase: a systemic review. Nutrition & diabetes. 2019; 9(1):23. https://doi.org/10.1038/s41387-019-0092-y.
- Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990-2000). International Journal of Diabetes and Metabolism. 2006; 14(1):1-25. https://doi.org/10.1159/000497588.
- Unuofin JO, Lebelo SL. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: an updated review. Oxidative medicine and cellular longevity. 2020; (1):1356893.
- Egbuna C, Awuchi CG, Kushwaha G, Rudrapal M, Patrick-Iwuanyanwu KC, Singh O, Odoh UE, Khan J, Jeevanandam J, Kumarasamy S, Chukwube VO. Bioactive compounds effective against type 2 diabetes mellitus: a systematic review. Current Topics in Medicinal Chemistry. 2021; 21(12):1067-1095.
- Bogle, IDL, Mendes MF. Evaluation of the effects and mechanisms of bioactive components present in hypoglycemic plants. International Journal of Chemical and Biomolecular Science. 2015; 1(3):167-178.
- Salehi B, Ata A, V. Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Valere Tsouh Fokou P, Kobarfard F, Amiruddin Zakaria Z, Iriti M. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019: 9(10):551.
- Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H. Antioxidant phytochemicals against type 2 diabetes. British Journal of Nutrition. 2008; 99:109–117. https://doi. org/10.1017/S000711450896579X.
- Ishnava KB, Metisariya DM. In vitro study on α-amylase inhibitory activity of selected ethnobotanical plant extra its and its herbal formulations. International Journal of Pharmacognosy and Chinese Medicine. 2018; 2(3):000136.
- He JH, Chen LX, Li H. 2019. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia. 2019;134: 270-289.
- Silva KB, Pinheiro CT, Soares CR, Souza MA, Matos-Rocha TJ, Fonseca SA, Pavão JM, Costa JG, Pires LL, Santos AF. Caracterização fitoquímica, potencial antioxidante e atividade antimicrobiana de Averrhoa carambola L. (Oxalidaceae) frente a patógenos multirresistentes. Brazilian Journal of Biology. 2020; 81(3):509-15. https://doi.org/10.1590/1519-6984.220259.
- Le DKT, Danova A, Aree T, Duong TH, Koketsu M, Ninomiya M, et al. α-Glucosidase Inhibitors from the Stems of Knema globularia. Journal of Natural Products. 2022; 85(4): 776–786. doi: 10.1021/acs.jnatprod.1c00765.
- Sgariglia MA, Garibotto FM, Soberón JR, Angelina, EL, Andujar SA, Vattuone MA. Study of polyphenols from Caesalpinia paraguariensis as α-glucosidase inhibitors: Kinetics and structure–activity relationship. New Journal of Chemistry. 2022; 46:11044–11055. https://doi.org/10.1039/D1NJ04619E.
- Sheikh Y, Chanu MB, Mondal, G, Manna P, Chattoraj A, Chandra DD et al. Procyanidin A2, an anti-diabetic condensed tannin extracted from Wendlandia glabrata, reduces elevated G-6-Pase and mRNA levels in diabetic mice and increases glucose uptake in CC1 hepatocytes and C1C12 myoblast cells. RSC Adv. 2019; 9:17211–17219. DOI: 10.1039/c9ra02397.
- Chen K, Liu XQ, Wang WL, Luo JG, Kong LY. Taxumarienes A–G, seven new α-glucosidase inhibitory taxane-diterpenoids from the leaves of Taxus mairei. Bioorg. Chem. 2020; 94:103400. https://doi.org/10.1016/j.bioorg.2019.103400.
- Salari-Moghaddam A, Nouri-Majd S, Keshteli AH, Emami F, Esmaillzadeh A, Adibi P. Association between dietary total antioxidant capacity and diet quality in adults. Frontiers in Nutrition. 2022; 9:838752. doi: 10.3389/fnut.2022.838752.
- Kashtoh H, Baek KH. Recent updates on phytoconstituent alpha-glucosidase inhibitors: An approach towards the treatment of type two diabetes. Plants. 2022; 11(20):2722. https://doi.org/10.3390/plants11202722.

 ETFLIN
Notification
ETFLIN
Notification







