Quality Control of Herbal Drug (Paxherbal Bitter Tea) Via Thin-Layer Chromatography and Phytoconstituent Analysis
by Tunde Ayobami Owolabi ★ , Emmanuel Amodu, James Danga
Academic editor: Garnadi Jafar
Sciences of Pharmacy 2(3): 211-215 (2023); https://doi.org/10.58920/sciphar02030115
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
23 Jul 2023
25 Aug 2023
26 Aug 2023
28 Aug 2023
Abstract: The effectiveness of any herbal medication depends on the presence or absence of pharmacologically active phytocompounds. These ingredients are significantly affected by the quality control system espoused during and after manufacturing processes and the operation of such pharmaceuticals. Thin-layer chromatography (TLC) is a quality control parameter suitable to identify and codify substances predicated on their distinctiveness and uniformity, giving an identity for such substances. Paxherbal Bitter Tea is a polyherbal medicine harnessed as an anti-diabetes medicine. This study assessed the distinguishable phytoconstituents present and evolved attribute profiles (TLC) for the herbal (Paxherbal Bitter Tea) to serve as a quality control check during manufacturing for consistency and market identity. Qualitative phytochemical and chromatographic assays were carried out employing standard methodologies. The qualitative test revealed the presence of saponin, steroids, reducing sugar, cardiac glycoside, and terpenoids, and the finger-print chromatograms after progression with n-hexane: ethyl acetate (9:1) flaunted three dissimilar elements under ultraviolet light at 365 nm and five spots, when dotted with 20% methanolic sulphuric acid under visible light. In this study, we developed an identity profile for Paxherbal Bitter Tea via its unique chemical biographies, which can be exercised in verifying the quality and consistency of the herbal product.
Keywords: Bioactive constituentsQuality controlPaxherbal Bitter TeaThin-layer chromatography
Introduction
Throughout history, healing plants have been applied in burdensome old, newly coming maladies and ails menacing the well-being and actuality of man on the earth. Correspondent conditions encompass the transmittable ones caused by microbes and non-transmittable and life-menacing ones like diabetes, hypertension, and various diseases (1). Traditional medicine-producing companies have been regarded as thriving in managing several infections and chronic conditions with the advantage of being detailly natural (2). Plants have legion bioactive principles like tannins, alkaloids, and steroids (3).
The aggrandizing public importunity for natural medicines has resulted in hyped merchantable exercise and output of these medicines. This has piloted growing interest in guaranteeing the consistency and quality of herbal drugs. To effectively attune the quality of raw materials, processing of stuff, and final productions, it has become imperative to develop reliable, special, and sensitive quality control checks. Thin-layer chromatography (TLC) has arrays of mileage in these regards. The methodology is simple, cost-operative, and adaptable. The usages of TLC in quality control of plant evaluation include attribute profiling for the evaluation of chemical constituents of an extract and quantitative assay of markers in plant drugs (4).
Paxherbal Bitter Tea is a polyherbal product from Nigeria, listed by the federal government of Nigeria as safe with REG. NO. A7-1982L, exercise as an adaptogenic, antimicrobial, anti-diabetes medicine. It is packaged in a box of thirty teabags, sold and allotted within and outside Nigeria for over 25 years. The medicine is offered as a tea bag (2.0 g) with a transparent nylon envelope in a tinderbox square box. An aromatic smell and a bitter taste characterize the tea. It consists of well-delved medicinal plants, which the most active ones include but are not limited to Bitter leaves (Vernonia amygdalina) and Pawpaw leaves (Carica papaya). It has been established that the applied natural exertion of any plant is the tracts of the phytochemicals contained in such a plant (1).
In the headway of our standardization of herbal medicines (5), we aimed at establishing fast and sensitive parameters for herbal drugs as a means of checkmating identity and quality control measures by evaluating the phytoconstituents and the TLC profile of Paxherbal Bitter Tea as summarised in Figure 1.
Experimental Section
Chemicals
The chemicals or reagents used in this work include concentrated sulphuric acid (H2SO4), ferric chloride (FeCl3), chloroform, ethyl acetate, methanol, n-hexane, hydrochloric acid (HCl), copper sulfate, acetic acid anhydride, all are of analytical grade (BDH chemicals, England), analytical pre-coated TLC plates (Sigma Aldrich, Germany) and the water employed was dual glass distilled.
Extraction of the Drug for
Phytochemical Screening
A kit bag of Paxherbal Bitter Tea from five distinguishable production batches was obtained from different accredited marketers (Auchi, Ekpoma, Uromi, Irrua, and Benin City, all in Edo State, Nigeria) of the drug. Each package was treated independently: 5 Teabags (10 g of tea content) were stint open, and the powder medicine was poured into a clean, dried beaker, weighed, and infused.
Concisely, 10 g of this substance was macerated in warm water (40°C) for 45 mins with constant agitation. This was screened employing a muslin textile, and the filtrate was allowed to cool and harnessed for the assay. The extracted filtrate was sampled for the presence and absence of bioactive composites by employing the standard procedures (6, 7) as simplified in another study (3).
Extraction for TLC
Five tea pokes of Paxherbal Bitter Tea (10 g) from five distinguishable product batches were extracted independently with 50 mL of absolute ethanol in a water bath maintained at 50°C for 15 mins. The admixture was screened through a muslin textile. The consequential filtrate was partitioned between 50 mL chloroform and 50 mL water. The chloroform phase was collected for the TLC assay for the five nonidentical batches numbered 1, 2, 3, 4, and 5, respectively.
The TLC was performed on an analytical pre-coated TLC plate (Silica gel, 60 F254, Sigma Aldrich, Germany). Samplings (1- 5, defining nonidentical product batches) were lumbered with a micro-capillary tube on the TLC plate and developed in a tank (Shandon Southern T.L.C Chromatank, Unikit) with mobile phase; n-hexane - ethyl acetate (9:1).
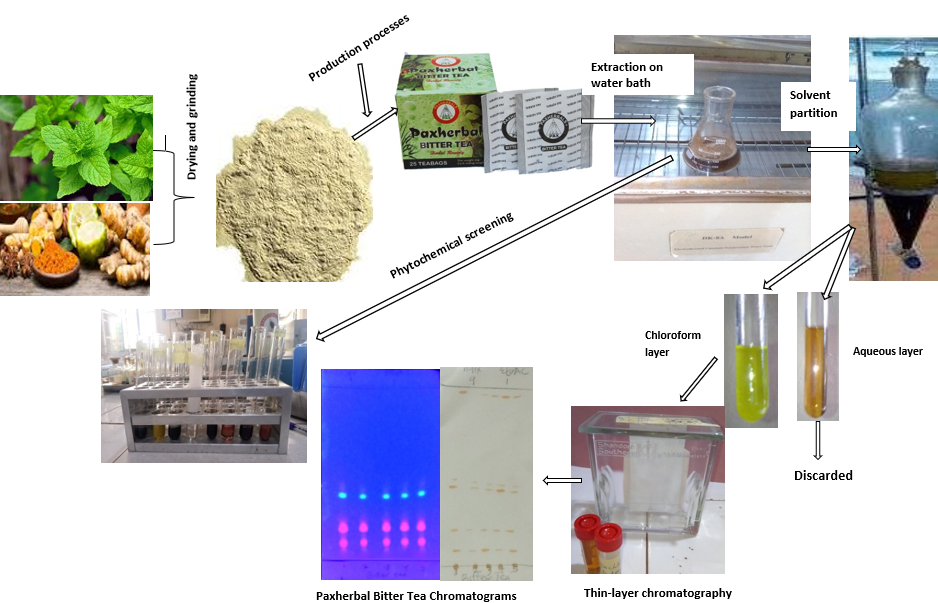
Figure 1. Flowchart of the methodology.
Table 1. Paxherbal Bitter Tea chromatograms under UV light (365 nm).
|
Components |
Color |
Rf (cm) |
|
1 |
Light green |
0.4000 |
|
2 |
Pink |
0.2375 |
|
3 |
Pink |
0.1125 |
Table 2. Paxherbal Bitter Tea chromatograms in visible light (with 20% methanolic sulfuric acid spray).
|
Components |
Color |
Rf (cm) |
|
1 |
Brown |
0.9750 |
|
2 |
Brown |
0.5250 |
|
3 |
Brown |
0.4875 |
|
4 |
Brown |
0.2500 |
|
5 |
Brown |
0.0375 |
Observation of Separation
Plates were viewed under 365 nm UV light (ZF-1, Niusiwen UV lamp, China) dotted with 20% methanolic sulphuric acids, heated at 105°C for 30 mins, and viewed under visible light (5).
Recording Chromatograms
Luminescence and non-fluorescence under UV light were recorded with a digital camera (Tecno Camon 20 CAMERA, Rear Triple (64 MP, f/1.7, 25mm (wide), 1/1.7", 0.8µm, PDAF2 MP, f/2.4, (depth) QVGA) and visualization was improved by changing discrepancy, intensity, and brightness employing picture editing software like as Microsoft Picture Editor (5).
Results and Discussion
Thin Layer Chromatography
Herbal drugs, be they mono or poly-herbal, are known to be a mixture of different constituents with established synergistic and or antagonistic effects. A standard quality analytical method is vital to effectively coordinate the quality of raw materials, processing, and the final output. Thin-layer chromatography is a widely accepted, fast technique for separating a mixture of compounds (8). The stationary phase consists of a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat carrier like a glass plate, a thick aluminium foil, or a plastic sheet. TLC has certain advantages over other forms of chromatographical techniques. Separation of constituents can be achieved more rapidly with smaller quantities of the mixture. The separated spots are usually more compact and more clearly demarcated from one another, and the nature of the film is often such that drastic reagents, such as concentrated sulphuric acid, can be used to locate separated constituents (9). Below are the obtained Rf values of the thin-layer chromatography.
After the TLC plates were viewed for fluorescent components, the dried chromatographic plates were subjected to universal chemical derivatizations, with 20 % alcoholic sulfuric acid being a general spraying reagent for natural products. This enables the visualization of other non-fluorescent constituents (10), as revealed in Table 2 below.
Less polar constituents move faster on the TLC plate, while more polar ones move slower (11). From Table 3's phytochemical results, terpenoids and steroids (Rf 0.9750 cm and 0.5250 cm) are less polar, while saponin, glycosides, alkaloids, tannins, and starch are polar. This suggests that the TLC plate's main constituents (less polar) are likely terpenoids and steroids, matching values in a previous study (12). The later constituents (more polar) include saponins, alkaloids, and tannins (Rf 0.4875, 0.2500, 0.0375 cm).

Figure 2. Chromatograms of Paxherbal Bitter Tea on silica gel GF254 with n-hexane:ethyl acetate (9:1) solvent system. Note: (A) viewed under ultraviolet light at 365 nm and (B) sprayed with 20% methanolic H2SO4 and viewed under visible light.
Three emphatic elements were observed under UV light with separate, distinct colors, as pictured in Figure 2a. These elements are the fluorescent ones and thus were obtained at a wavelength of 365. Their color and Rf data are given in Table 1. However, five elements were observed after the plates were treated with 20% methanolic sulphuric acid under visible light, as presented in Figure 2b and Rf data in Table 2. The mileage of TLC in the quality control of herbal medicines can't be overemphasized (13, 14). Its excellence includes but is not confined to low cost, simplicity, and reproducibility. The appearance of chromatograms in familiar, nonidentical bands can be useful for identifying and authenticating medicinal herbs.
The TLC biographies of the drug studied (Paxherbal Bitter Tea) shown in Figures 2a and b unveiled that the drug examined has some fluorescent and non-fluorescent elements with aligned bands and the same Rf valuations for all the nonidentical product batches. All identifications in the TLC are hung on a comparison of the migration distances (Rf data) and the color of the spots between the sample when the TLC plate is dotted with a peculiar chromogenic reagent. The quality of the assay depends on the accurate positioning of the samplings employed in the TLC (14).
Phytochemical Screening Result
and Discussion
Out of the ten groups of phytoconstituents screened for in the filtrate of the medicine, the results attained are offered in Table 3 below.
Table 3. Results of the qualitative phytochemical screening of Paxherbal Bitter Tea.
|
Phytoconstituents |
Batch 1 |
Batch 2 |
Batch 3 |
Batch 4 |
Batch 5 |
|
Cardiac Glycoside |
- |
- |
- |
- |
- |
|
Saponin |
+ |
+ |
+ |
+ |
+ |
|
Tannin |
- |
- |
- |
- |
- |
|
Phlobatannin |
+ |
+ |
+ |
+ |
+ |
|
Flavonoid |
- |
- |
- |
- |
- |
|
Steroid |
+ |
+ |
+ |
+ |
+ |
|
Alkaloid |
+ |
+ |
+ |
+ |
+ |
|
Reducing sugar |
+ |
+ |
+ |
+ |
+ |
|
Terpenoid |
+ |
+ |
+ |
+ |
+ |
|
Polysaccharide/Starch |
+ |
+ |
+ |
+ |
+ |
Note: (+) means present, and (-) means absent.
Farombi and Owoeye (15) have reported a similar trend of obtained phytoconstituents in Vernonia amygdalina, while Singh et al. (14) have reported similar phytoconstituents of Carica papaya leaves with slight differences. These differences could be a result of material combination and processing. The therapeutic exercises of medicinal plants have been proven to be the function of the type and nature of phytochemicals present in them (1, 11-14). The phytochemicals present in the studied medicine (Table 3) contain alkaloids and terpenoids. These constituents have been described to parade a wide range of exercises encompassing hypoglycemic (1, 18), which is the exertion denoted by the manufacturer of the drug delved (Paxherbal Bitter Tea).
It is also known that alkaloids are active constituents against hyperglycemia, high blood pressure, and anti-stress. Saponins are also used as tranquilizers (19). Terpenoids have been established to be potent antimicrobial agents, and saponins have been known to be synthesized by plants in response to microbial attacks. Hence, they are effective antimicrobial substances against a wide array of microorganisms.
Conclusion
From the present studies, it can be concluded that phytoconstituents of the examined herbal drug (Pax Herbal Bitter Tea) are confined to saponin, cardiac glycosides, phlobattanin, reducing sugar, steroid, and terpenoid, which many researchers have reported having direct biological activities (adaptogenic, antimicrobial, and anti-diabetes) as claimed by the manufacturer. The product also contains five emphatic spots on the TLC plate of silica gel GF254 with n-hexane:ethyl acetate (9:1) solvent systems, sprayed with 20% methanolic H2S04, viewed under visible light and three constituents when viewed at 365 nm as its quality parameters for identity and consistency of the herbal drug.
Declarations
Acknowledgment
We acknowledge the support of Pax Herbal Clinic and Research Laboratories management for providing the drug (Paxherbal Bitter Tea) for the study.
Ethics Statement
Not applicable.
Data Availability
Information related to the product can be accessed at https://www.paxherbals.net/#. Data related to the study is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Owolabi TA, Ayinde BA. Bioactivity guided isolation and characterization of anti-cancer compounds from the Stem of Musanga cecropioides R. Br. Ex Tedlie (Urticaceae). Journal of Pharmacognosy and Phytochemistry. 2021; 10(6): 292-296.
- Nigeria Natural Medicine Development Agency, (NNMDA). Federal Ministry of Science and Technology 9, Kofo Abayomi Street, Victoria Island, Lagos, Nigeria. 2009; p. 1-49.
- Owolabi TA, Salome OE. Quantification of bioactive constituents of mistletoe leaves (Tapinanthus globiferus a. rich) from four different host plants in the EWU community. International journal of advance chemistry. 2021: 10 (1) 1-4.
- Mohammad A, Bhawani SA, Sharma S. Analysis of herbal products by thin-layer chromatography: a review. Int J Pharma Bio Sci. 2010:1: 1-50.
- Owolabi, T., Osaretin, D., and Eyinayan, B. Bioactive composition and TLC profile data on Pax Herbal Malatreat Tea. Drug Analytical Research. 2022; 6(1), 35–39.
- Shah B.N and Seth A.K. Textbook of Pharmacognosy and Phytochemistry. Mosby, Saunders, Churchill Livingstone, Butterworth Heinemann and Hanley & Belfus are the Health Science imprints of Elsevier. 2010.
- Harborne JB. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. Chapman and Hall, London. 1973; p. 279. https://doi.org/10.22456/2527-2616.125038.
- Laurence D, Juraj H, Rene´ Lafont. Chromatographic procedures for the isolation of plant steroids. Journal of Chromatography A. 2002; 935 (201) 105–123.
- Trease GE, Evans WC. Pharmacognosy. 15th Ed. London: Saunders Publishers. 2002; pp. 42–44.
- Agatonovic-Kustrin S, Kustrin E, Gegechkori V, Morton DW. High-Performance Thin-Layer Chromatography Hyphenated with Microchemical and Biochemical Derivatizations in Bioactivity Profiling of Marine Species. Mar Drugs. 2019 Mar 3;17(3):148. doi: 10.3390/md17030148.
- Durón RR, Almaguer LC, Garza-Juárez AJ, Cavazos MLS, De-Torres NW. Development and validation of thin-layer chromatographic methods for quality control of herbal products. Acta Chromatogr. 2009; 21: 203-215.
- Asante IK, Owusu E, Essilfie MK, Kwarteng M and Amuzuah O. Phytochemical investigation and thin layer chromatography of methanolic extracts of some selected grain legumes. Journal of Pharmacognosy and Phytochemistry 2016; 5(3): 240-244.
- Reich EA, Blatter BM. “TLC for the analysis of herbal drugs.” GIT laboratory journal Europe 7. 2003; 262-263.
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr J Tradit Complement Altern Med. 2011;8(1):1-10. Epub 2010 Oct 2. PMID: 22238476; PMCID: PMC3218439.
- Farombi EO, Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health. 2011 Jun;8(6):2533-55. doi: 10.3390/ijerph8062533. Epub 2011 Jun 23. PMID: 21776245; PMCID: PMC3138040.
- Singh SP, Kumar S, Mathan SV, Tomar MS, Singh RK, Verma PK, Kumar A, Kumar S, Singh RP, Acharya A. Therapeutic application of Carica papaya leaf extract in the management of human diseases. Daru. 2020 Dec;28(2):735-744. doi: 10.1007/s40199-020-00348-7.
- Ajebli M, Khan H, Eddouks M. Natural Alkaloids and Diabetes Mellitus: A Review. Endocr Metab Immune Disord Drug Targets. 2021;21(1):111-130. doi: 10.2174/1871530320666200821124817.
- Sok Yen F, Shu Qin C, Tan Shi Xuan S, Jia Ying P, Yi Le H, Darmarajan T, Gunasekaran B, Salvamani S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid Based Complement Alternat Med. 2021; 8;2021:2057333. doi: 10.1155/2021/2057333.
- Ilozue N.M., Ikezu U.P. & Ugwu Okechukwu PC. Anti-Microbial and Phytochemical Screening of the Seed Extracts of Persea Americana (AVOCADO PEAR). IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS). 2014, Mar-Apr. Volume 9, Issue 2 Ver. VI, PP 23-25.

 ETFLIN
Notification
ETFLIN
Notification









