RESEARCH ARTICLE
Home>Article>10.58920/sciphar02040045
Graphical Abstract
Home>Article>10.58920/sciphar02040045

RESEARCH ARTICLE
Academic Editor: Mohd Shahezwan Abd Wahab
Sciences of Pharmacy|Vol. 2, Issue 4, pp. 249-255 (2023)
Views
Downloads
Shares
Received
Oct 31, 2023Revised
Nov 24, 2023Accepted
Nov 28, 2023Published
Nov 29, 2023The clinical progression from Human Immunodeficiency Virus (HIV) infection to Acquired Immunodeficiency Syndrome (AIDS) involves the gradual destruction of CD4 cells vital for immune defense. Globally, there are 37.7 million HIV cases, with 427,201 reported in Indonesia by March 2021, including 131,417 AIDS cases (1). The advent of antiretroviral (ARV) treatment, available since around 1996, marked a significant advancement in the management of HIV, helping to control the virus and prevent the onset of AIDS (2). However, ARVs are not without their challenges, as they can give rise to adverse drug reactions (ADRs) – unwanted responses occurring at therapeutic doses (3).
ADRs refer to harmful and unintended responses or effects that occur after the administration of a medication at regular therapeutic doses (4). ADRs are a significant concern in healthcare, as they can lead to various issues, including patient discomfort, treatment interruptions, increased healthcare costs, and, in severe cases, morbidity and mortality (5). For example, patients treated at the Emergency Room in Gunung Jati Cirebon, common ADRs encompass a range of symptoms, such as headaches (22.1%), fatigue (6.8%), pain (9.3%), itching (14.4%), nausea (20.1%), diarrhea (7.2%), lipodystrophy (2.0%), skin rash (11.3%), skin discoloration (1.6%), neuropathy (1.6%), and sleep disturbances (3.6%) (6).
Understanding the risk factors associated with these ARV-related side effects is of paramount importance in the ongoing effort to enhance patient care. Previous research has illuminated numerous cases of ADRs, offering insights into potential causes and solutions for these undesirable effects (7-9). The primary objective of our study was to delve deeper into the risk factors and ADRs induced by ARVs and, in doing so, provide invaluable information to improve the management of HIV/AIDS patients. Through a comprehensive approach involving a detailed examination of medical records and the application of rigorous statistical methods, we seek to pinpoint the primary factors and adverse drug reactions (ADRs) among HIV/AIDS patients at Undata Regional Hospital in Palu, which was the hospital with the highest number of HIV/AIDS patients. This investigation is particularly significant as the incidence of HIV/AIDS has been on the rise and is associated with the increasing prevalence of homosexual practices (10). Our research endeavors to optimize treatment strategies, minimize side effects, and ultimately elevate the overall quality of life for individuals living with HIV/AIDS.
The present study employed a cross-sectional observational approach with retrospective data collection. This research design falls under the quantitative research category, aiming to assess the incidence of Adverse Drug Reactions (ADRs) and explore potential correlations between ADR incidence and various risk factors. This research was conducted from March to April 2021 at Undata Regional General Hospital, Central Sulawesi Province. This study was approved by Ethical Committee of Universitas Tadulako with ethical approval number of 4129/UN28.1.30/KL/2023.
To gather the necessary data, we conducted an extensive review of medical records, drawing upon a robust and representative sample of HIV/AIDS patients at Undata Regional Hospital in Palu. This method allowed us to access a substantial volume of historical patient data, facilitating a comprehensive analysis of ADR cases. Our study primarily focused on HIV/AIDS patients, who form a vulnerable and medically complex population. We recognized the importance of including this group due to the necessity of antiretroviral therapy (ART) for managing HIV infection and the potential for ADRs associated with long-term ART use. The study population included individuals from diverse backgrounds, reflecting the local demographic diversity in Palu.
To be eligible for the study, participants had to meet specific criteria, including falling within the age range of 18 to 65 years, receiving a confirmed diagnosis of HIV/AIDS, and possessing complete and accessible medical records that contained essential information on treatment regimens, clinical history, and ADR reports. Patients outside the defined age range, those with incomplete or inaccessible medical records, and individuals with severe comorbid conditions that could significantly impact the assessment of ADRs were excluded from the study.
The exclusion of individuals with severe comorbid conditions from the study was based on the need to ensure that the study results were not unduly influenced by factors unrelated to HIV/AIDS or that could complicate the assessment of adverse drug reactions (ADRs). Factors that we considered when defining severe comorbid conditions include (a) medical severity including its impact on overall health and the potential for complications; (b) treatment complexity managing the comorbid condition and the potential for interactions with HIV/AIDS treatments; (c) impact on study outcome; (d) lacking of comorbid information; and (e) other clinical judgments considering factors such as prognosis, symptomatology, and overall health status.
A fundamental component of this study was the investigation of potential risk factors contributing to ADRs among HIV/AIDS patients. We assessed various factors, such as demographic information, medical history, drug regimens, and lifestyle choices. This allowed us to explore correlations between these factors and the occurrence of ADRs. Univariate analysis to describe the distribution of each variable observed including, gender, age, HIV/AIDS patients parameters, lifestyle, comorbidities, incidence of ADRs, and types of ADRs.
The research data pertaining to gender, as depicted in Figure 1, revealed a notably higher prevalence of HIV/AIDS infection among males, totaling 108 patients (75%) in comparison to their female counterparts. This discrepancy can be attributed to the transmission of HIV among men, particularly some homosexual men who engage in unprotected anal intercourse, which may subsequently be transmitted to their female partners. Furthermore, it is important to note that men who have sex with men may also engage in risky sexual practices with women, significantly elevating the risk of HIV/AIDS transmission (11).



The age distribution depicted in Figure 1 reveals a predominant occurrence of HIV/AIDS among patients aged 18 to 35 years, constituting 95 cases (75%). This is followed by individuals aged 36 to 45 years (16%) and those aged 46 to 65 years (9%). This trend can be attributed to the higher likelihood of engaging in unsafe sexual practices at a younger age, which significantly elevates the risk of HIV transmission. Such unsafe sexual behavior encompasses engaging in sexual relations with multiple partners without the use of condoms (12, 13). Additionally, it is important to consider the potential influence of experimentation and the desire to use non-sterile syringes in the context of drug consumption (14).
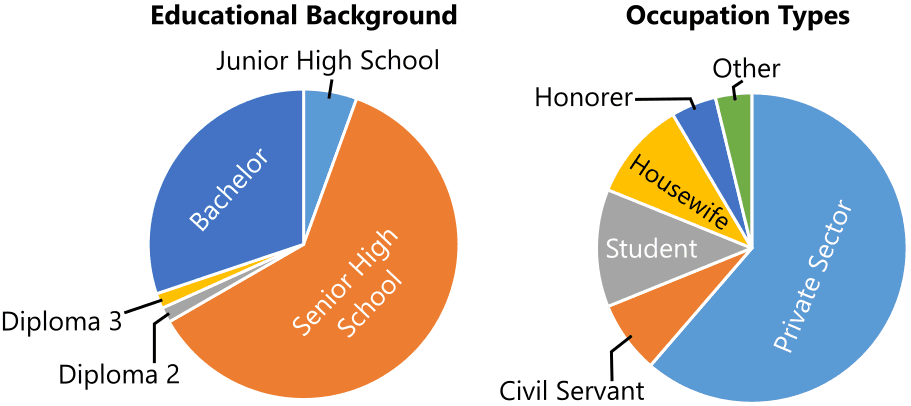
The study results concerning the education level, as illustrated in Figure 2, indicate a significant proportion of HIV/AIDS infections among individuals with a high school education, comprising 77 patients (61%). This trend can be attributed to the relatively lower level of awareness and knowledge among adolescents about the risks and transmission of HIV/AIDS (15). This lack of awareness significantly contributes to a higher occurrence of HIV/AIDS. This is primarily because adolescents are more likely to engage in unprotected sexual activity and use non-sterile syringes for drug use, given the greater opportunities available to them (16).
As depicted in Figure 2, the data regarding employment status reveals a higher representation of private employees, with 65 patients (61%). This observation can be attributed to the nature of private employment, which often involves high mobility outside the home. Various factors associated with this mobility may influence individuals to engage in risky sexual behavior or commercial sex (17). Furthermore, individuals who are employed and have their own income are often driven by the need to satisfy their desires, such as purchasing sex, a behavior that renders them more vulnerable to HIV infection, in addition to substance abuse (18).
The research findings as shown in Figure 3 reveal that the most significant proportion of HIV/AIDS infections pertained to individuals with an unmarried marital status, comprising 87 patients (71%). Marital status is recognized to have an impact on the sexual behavior of both married and unmarried men. Married men tend to engage in responsible and safe sexual practices with their partners, as they are less likely to seek adverse consequences from their sexual encounters. In contrast, unmarried individuals are theoretically more inclined to seek sexual satisfaction through engagements with female sex workers, thereby exhibiting a higher propensity for engaging in risky sexual behavior (19).
The primary risk factor for HIV/AIDS infection among patients is engaging in sexual intercourse with men, specifically homosexual encounters, accounting for 73 patients (58%). This heightened risk is attributed to the prevalence of anal intercourse within male-to-male sexual relationships, a practice associated with the highest risk of HIV/AIDS transmission. The physiological characteristics of the anus are not conducive to sexual intercourse, making it prone to injury during anal sex and facilitating the entry of HIV into the body (20, 21).
|
Clinical Data |
Number of Patients |
Percentage (%) |
|
Grade 1 |
8 |
6 |
|
Grade 2 |
44 |
35 |
|
Grade 3 |
54 |
43 |
|
Grade 4 |
20 |
16 |
The results from Table 1 regarding the stages of HIV/AIDS indicate that the majority of patients were at stage 3, comprising 50 patients (39%). The progression through the four clinical stages of HIV/AIDS corresponds to a decline in CD4 count, signifying an increase in the viral load within the patient's body (22).
Based on opportunistic infections presented in Table 2 reveals that candidiasis affected a total of 35 patients (33%). This occurrence can be attributed to the fact that HIV patients with low CD4 counts have compromised immune systems that are unable to combat opportunistic infections, including fungal infections. HIV infection can alter the prevalence of fungal diseases, resulting in a correlation between a higher incidence of fungal infections and a lower CD4 count (23, 24).
|
Opportunistic Infections |
Number of Patients |
Percentage (%) |
|
TBC |
19 |
18 |
|
Condyloma |
4 |
4 |
|
Dermatitis |
2 |
2 |
|
Diare Cryptosporidia |
14 |
13 |
|
Hepatitis |
2 |
2 |
|
Herpes |
13 |
|
Number of CD4 |
Number of Patients |
Percentage (%) |
|
<200 sel/mm3 |
27 |
21 |
|
200-500 sel/mm3 |
10 |
8 |
|
>500 sel/mm3 |
2 |
2 |
|
Nothing |
87 |
69 |
In Table 3, the data concerning the initial CD4 counts indicates that the majority of results were reported as "none," encompassing 87 patients (69%). This outcome can be attributed to the unavailability of the necessary reagents or materials required to perform CD4 tests within the hospital. As a result, patients are compelled to seek CD4 testing outside the hospital, incurring a relatively high cost for the procedure. Due to the financial constraints associated with these expenses, not all patients undergo the CD4 test.
Table 4 displays the treatment outcomes, indicating that the most commonly employed treatment regimen is the 3FDC ARV combination, which was administered to 123 patients (96%). This preference is primarily due to the fact that the Tenofovir-Lamivudin-Efavirenz ARV drug combination has been designated as the first-choice combination for HIV/AIDS patients (25). This regimen aligns with the recommendations set forth by the World Health Organization (WHO) and the Indonesian Ministry of Health as the current first-line ARV therapy. It comprises a combination of two classes of Nucleoside Reverse Transcriptase Inhibitors (NRTIs) and one Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI), which are formulated as a fixed-dose combination (FDC) in a single ARV tablet.
|
ARV Combination |
Number of Patients |
Percentage (%) |
|
3 FDC |
123 |
96 |
|
Duviral-EFV |
1 |
1 |
|
Evafirenz |
1 |
1 |
|
Nevirapine |
1 |
1 |
The NRTIs function by undergoing three stages of intracellular conversion, involving the addition of three phosphate groups. Subsequently, they compete with natural nucleotides and inhibit Reverse Transcriptase, thereby inhibiting the conversion of RNA to DNA. Additionally, NRTIs impede DNA elongation. In contrast, the NNRTI class of drugs operates by inhibiting the reverse transcriptase enzyme through conformational changes that render the enzyme inactive. The ARV tablet contains the following doses: Tenofovir 300 mg, Lamivudin 300 mg, and Efavirenz 600 mg (26).
Patients received a standardized treatment in the form of a fixed dose combination, showing limited variability that hinders the assessment of its correlation with adverse drug reactions (ADRs). Despite the constrained information on factors associated with ADRs, this report serves as valuable information for patients to be mindful of during their activities, aiding in the prevention of potential harm and providing a useful monitoring tool, particularly for individuals undergoing HIV/AIDS treatment in Palu City.
|
Side Effects |
Number of Patients |
Percentage (%) |
|
Fever |
17 |
6 |
|
Dizziness |
120 |
47 |
|
Nausea |
30 |
12 |
|
Vomiting |
17 |
7 |
|
Sleeplessness |
39 |
15 |
|
Easily hungry |
2 |
According to Table 5, which categorizes the types of side effects experienced by HIV/AIDS patients, dizziness was the most prevalent, affecting 120 patients (47%). This occurrence is primarily attributed to non-specific side effects associated with the central nervous system, such as dizziness, vertigo, headache, and nausea, which are common with Antiretroviral Drugs (ARVs) (27, 28). Nausea, for instance, stems from the stimulation of the vomiting center in the medulla oblongata. Elevated levels of ARVs in the bloodstream may lead to side effects like hypersensitivity reactions, often characterized by itching and rashes, as indicated by studies based on pharmacogenomic data (29, 30).
In summary, the study at Undata Regional Hospital highlights a higher prevalence of HIV/AIDS among males engaged in risky sexual practices, particularly unprotected anal intercourse. Young adults (18-35 years) are disproportionately affected, emphasizing the need for interventions targeting unsafe sexual behavior and drug use in this demographic. Lower education levels and certain occupations contribute to increased vulnerability. Unmarried individuals exhibit a higher propensity for risky sexual behavior. Clinical data indicate a substantial number of patients at advanced HIV/AIDS stages, emphasizing the importance of early detection. Opportunistic infections, notably candidiasis, are common due to compromised immune systems. Challenges in accessing CD4 testing impact data availability. The prescribed treatment regimen aligns with guidelines, and side effects, such as dizziness, underscore the need for careful monitoring. These findings provide valuable insights for targeted interventions and improved care for individuals with HIV/AIDS.
The authors declare no conflicting interest.
The unpublished data is available upon request to the corresponding author.
The study was approved by Ethical Committee of Universitas Tadulako with ethical approval number of 4129/UN28.1.30/KL/2023.
Not applicable.
12
|
Candidiasis |
35 |
33 |
|
Toxoplasmosis |
5 |
4 |
|
No Interaction |
13 |
12 |
1
|
Low Hb |
2 |
1 |
|
Weakness |
2 |
1 |
|
Rash |
23 |
9 |
|
Headache |
3 |
1 |