Enhanced Ability of Agarwood Leaves (Aquilaria malaccensis Lam.) Ointment as Wound Healing to Heal Second-Degree Burns in Rats
by Yesi Desmiaty , Ni Made Dwi Sandhiutami , Fahleni Fahleni , Agnes Griselda, Amalia Zahra Apriliana ★
Academic editor: Muhammad Sulaiman Zubair
Sciences of Pharmacy 3(1): 51-60 (2024); https://doi.org/10.58920/sciphar0301214
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
21 Jan 2024
09 Mar 2024
09 Mar 2024
14 Mar 2024
Abstract: Agarwood leaves (Aquilaria malaccensis Lam.) contain chemical substances such as alkaloids, flavonoids, and triterpenoids that contribute to the healing process of burns. This study aims to evaluate the wound healing activity of spray-dried extract from agarwood leaves formulated into an ointment for second-degree burns. The method involves extracting agarwood leaves through the decoction method, followed by spray drying, and subsequent evaluation of the extract. The prepared extract was then formulated into an ointment and tested for wound healing activity on 24 white rats of the Sprague-Dawley strain, divided into four groups, each consisting of 6 rats: negative control (ointment base), positive control (Betadine®), formula 1 (agarwood leaves extract ointment with 20% extract concentration - ALO-20), and formula 2 (agarwood leaves extract ointment with 30% extract concentration - ALO-30). Second-degree burns were induced by exposing the rats' backs to ferrous metal for 3 seconds and treating them for 14 days. Observations were made by assessing changes in burn diameter and scab formation. In this study, ALO-30 demonstrated superior activity. Scab formation was faster on day 3, and the burn diameter was reduced by day 7. The results indicated that ALO-30 led to a quicker reduction in wound diameter compared to ALO-20 and the negative control. Agarwood leaves extract ointment with a concentration of 30% (ALO-30) exhibited a more effective wound healing effect than the ointment with a 20% concentration (ALO-20).
Keywords: Agarwood leavesWound-healingHerbal ointmentSecond-degree burns
Introduction
Burns are particularly hazardous due to the potential for them to cause life-threatening or long-lasting harm, in addition to inflicting severe physical and mental anguish and financial expenditures (1). For that reason, wounds require treatment. Second degree burns need 7 to 20 days to heal (2). The wound healing process normally consists of four phases, hemostasis, inflammation, proliferation, and tissue remodeling or resolution. These phases and their biophysiological functions must occur in a precise sequence, at specific times, and continue for a specific duration at optimal intensity. There are many factors that can influence wound healing that interfere with one or more phases in this process, resulting in inappropriate or compromised tissue repair (3). Cleaning, cooling with flowing water or a cold compress, applying a topical agent, donning a sterile dressing, and restoring full function are all effective treatments for second-degree burns. Topical medications such as povidine iodine and topical heparin are also effective in the heal burns (2, 4, 5). Hence, it is imperative to advance innovative methodologies or treatments that not only halt the progression of burn wound infections but also potentially accelerate wound recovery while mitigating adverse effects.
Treatment doesn't always involve medicinal ingredients; rather, plants in our surroundings can also serve as remedies, harnessing the active compounds within them. There is currently a heightened demand in developing countries for herbal products as therapeutic drugs, owing to their superior efficacy, compatibility with the human body, sustained use as nutraceuticals, and treatment options without significant side effects (6-9). Moreover, these herbal medicinal products are deemed safer than synthetic pharmaceuticals due to their natural composition (9). One such plant with potential wound-healing properties is the agarwood leaves (Aquilaria malaccensis Lam.). Aquilaria spp. exhibits pharmacological activities such as antibacterial, antifungal, analgesic effects, antiasthma, cytotoxicity, anti-diabetes, antioxidant, and anti-inflammatory properties (10). Aquilaria sinensis, in particular, contains the active ingredient flavonoid glycoside, which acts as an anti-inflammatory agent by inhibiting the production of pro-inflammatory agents, specifically Reactive Nitrogen Species, produced by macrophage cell lines (11). Previous research has highlighted the presence of alkaloids, flavonoids, and triterpenoids compounds in agarwood leaves of the Aquilaria malaccensis species, demonstrating their role in the healing process of burn wounds (12, 13).
In this research, leaves were chosen as the plant part for examination, as phytochemical screening of ethanol, methanol, and water extracts from agarwood leaves across various species consistently revealed the presence of alkaloids, flavonoids, and triterpenoids (10). The leaves of agarwood were subjected to extraction using the decoction method with water as the solvent. Subsequently, the obtained extract underwent drying through a spray dryer, and the quality parameters of the extract were determined. The dried results were then formulated into an ointment, chosen for its prolonged contact time with the skin. Two different formulations of agarwood leaf extract ointment, with varying concentrations, were prepared. Activity tests were conducted on the burnt areas on the backs of rats. Observations included measuring the initial wound diameter and monitoring scab formation. The analysis results were then compared among the positive control, negative control, ALO-20, and ALO-30 formulations.
Experimental Section
Leaves of agarwood were extracted utilizing the decoction method with water as the solvent. The extracted material was subsequently subjected to drying through spray drying and then transformed into an ointment. The ointment, formulated from the extract of agarwood leaves, consisted of two different formulations with varying concentrations. Activity tests were conducted on the burned areas on the backs of rats. Observations involved measuring the initial wound diameter and monitoring scab formation. The analysis results were then compared with those of the positive control, negative control, ALO-20, and ALO-30.
Plant Material
The material used in this research was agarwood leaves obtained from Pangkalan Baru, Central Bangka, Bangka Belitung that were determined in the Herbarium Depokensis, Biota Collection Room, Department of Biology, Faculty of Mathematics and Science, University of Indonesia, Depok. With number of determination 402/UN2.F3.22/PDP.02.00/2021.
Materials
Test animal cages, induction equipment (ferrous metal), iron tongs, Bunsen burner, spirit lamp, shaving tools, ether hood, apparatus for preparing water extract of agarwood leaves, infrared thermometer, glass beakers, blender, stirring rod, analytical balance, rat food containers, rat drinking bottles, scissors, disposable gloves, tweezers, scalpels, jars, silicate crucibles, furnaces, ovens, vials, caps, spatulas, dropper pipettes, weighing paper, and apparatus for preparing agarwood leaf extract ointment were utilized in the experiment. The instruments included Karl Fischer titrator (870 KF Titrino Plus Metrohm), analytical balance (Shimadzu, ATY224), pH meter (Hanna Instrument HI 2211), and AAS spectrophotometer (Shimadzu AA-7000).
The materials employed in this study encompassed an ointment preparation with a 20% concentration of agarwood leaf extract (ALO-20), an ointment preparation with a 30% concentration of agarwood leaf extract (ALO-30), an ointment preparation without active substances using a simple ointment base according to D. Ghosh et al., 2019, as a negative control, and a market comparison product with the trade name Betadine® ointment as a positive control. Additionally, ether, 70% ethanol, ingredients for preparing agarwood leaf extract ointment, sterile gauze, plaster, and sterile cotton were included in the research materials.
Extract Preparation
Agarwood leaves were extracted utilizing the decoction method, involving boiling in water for 30 minutes at approximately ±90°C, followed by filtration. The filtrate underwent evaporation and drying processes using a spray dryer. The decoction method was chosen for extracting agarwood leaves due to its simplicity, especially in extracting water-soluble compounds like tannins, alkaloids, and flavonoids, which are abundant in agarwood leaves (14).
Extract Phytochemical Screening
The study encompassed the phytochemical screening of the simplicia, involving the identification of alkaloids, steroids, triterpenoids, flavonoids, saponins, quinones, tannins, and essential oil. Alkaloid testing was conducted using Mayer and Dragendorff reagents. Mayer's reagent resulted in a white sediment, while Dragendorff produced a brick-red sediment upon detecting alkaloid compounds in the sample. Steroids and triterpenoids were analyzed using Lieberman Buchard's reagent, leading to a color change to either red or green. The testing for flavonoids involved the production of a colored layer by combining magnesium powder and amyl alcohol. For saponin testing, the sample was shaken, and if foam persisted after the addition of HCl and settling, the solution was considered positive for saponin. To test for quinones, NaOH was added to the sample, resulting in a red color if positive. The presence of tannins was determined by employing the ferric (III) chloride reagent, yielding a blue hue. Coumarin content assessment involved observing the sample's fluorescence, which exhibited a green and blue hue in the presence of tannins. Essential oil analysis entailed heating the sample until a residual substance emitted a distinct odor (15).
Extract Non-specific Parameters
The determination of total ash content was conducted using the gravimetric principle, while the insoluble acid content was determined utilizing the same principle to assess the ash content that remained dissolved in diluted HCl. The water content was determined through the Karl Fischer titration technique. The mechanism of contraction in the loss on drying process is gravimetric in nature. The water-soluble compound was ascertained gravimetrically using a water-chloroform solvent, and the residue was obtained through a process identical to determining the ethanol-soluble compound content, where ethanol served as the solvent (16, 17).
Agarwood Leaves Extract Ointment Formulation
In the formulation process of this ointment, agarwood leaf extract was prepared with concentrations of 20% and 30%. The concentration of agarwood leaf extract was determined based on the findings of the research conducted by Aris in 2019 (18). The formulation followed a base described in the journal by D. Ghosh et al., 2019 (19), which consisted of a simple ointment base (5 g adeps lanae, 5 g hard paraffin, 5 g cetostearyl alcohol, and 85 g vaseline to make up 100g).
Table 1. Aquilaria malaccencis ointment formula.
|
Materials |
Formula (g) |
||
|
Placebo |
ALO-20 |
ALO-30 |
|
|
Agarwood leaves extract |
- |
20 |
30 |
|
Adeps lanae |
5 |
5 |
5 |
|
Hard paraffin |
5 |
5 |
5 |
|
Cestostearyl alcohol |
5 |
5 |
5 |
|
Vaselin |
ad 100 |
ad 100 |
ad 100 |
Agarwood Extract Ointment Evaluation
The assessment of the agarwood leaves extract ointment involved organoleptic examinations (observing shape, color, and odor) and visual homogeneity examinations at room temperature. This was achieved by applying the ointment to a slide, covering it with another slide, and observing whether the base was thoroughly mixed with other materials. The ointment preparations subjected to evaluation included a simple base negative control ointment, a 20% concentration agarwood leaves extract ointment (ALO-20), and a 30% concentration agarwood leaf extract ointment (ALO-30). Additionally, a comparative product, Betadine® ointment, was used as a positive control. The evaluation of the ointment preparations included organoleptic tests, homogeneity assessments, spreadability tests, and pH examinations.
Organoleptic Evaluation
Organoleptic examination was carried out at room temperature (25-30°C) visually on the appearance of the ointment which includes changes in shape, color and odor of the ointment preparation.
Homogenity Evaluation
The homogeneity assessment of the ointment preparation was conducted at room temperature (25-30°C) by applying the ointment to a glass surface. Subsequently, the glass surface was covered with another glass object to observe whether the base is uniformly mixed with the other ingredients, ensuring a smooth and even surface.
pH Evaluation
The pH assessment was performed utilizing a pH meter, wherein a pH 7 solution (echymolal phosphate buffer) and a pH 4 solution (potassium biphthalate buffer) were employed for calibration. Distilled water was used for rinsing, ensuring the complete submersion of the electrode tip until the digital reading stabilized, indicating readiness for measurement. The recorded pH value was then documented.
Spreadability Evaluation
The examination of the spreading ability of the ointment preparation was conducted 48 hours after its production. This was achieved by measuring the spreading diameter of 1 g of ointment between two glass boards measuring 8x8 cm, three minutes after application. The ointment was applied within a ring with an outer diameter of 55 mm, a thickness of 3 mm, and an inner diameter of 15 mm with a glass bottom. The Teflon ring was filled with ointment, smoothed with a spatula to obtain a flat surface without air bubbles. Subsequently, the ring was carefully lifted to allow the ointment to spread with a diameter of 15 mm and a thickness of 3 mm. The ointment preparation was then covered with a glass plate with a diameter of 8 cm, weighing 20 g. It was pressed with a weight of 28 g, left for 3 minutes, moved, and the diameter of the expanded ointment surface was measured using a caliper in mm. The calculation was performed using the formula: F = π × r2
Animal Testing
The research obtained ethical approval under the number KET-633/UN2.F1/ETIK/PMM.00.02/2021. A total of 24 white rats of the Sprague-Dawley strain were utilized, divided into four groups, each comprising six rats (Rattus norvegicus). The groups included a negative control (ointment base), positive control (Betadine®), formula 1 (ALO-20: 20% extract concentration ointment), and formula 2 (ALO-30: 30% extract concentration ointment). These rats were healthy, aged 2.5-3 months, and weighed between 200-250 grams, sourced from the Faculty of Animal Science, IPB (Institut Pertanian Bogor). The male white rats of the Sprague Dawley strain underwent an adaptation period, being housed in cages layered with husks that were changed every three days to maintain dryness. Additionally, daily care involved providing food and water.
Burns Making
Male white rats from the Sprague Dawley strain had their backs shaved the day before they were burned, then anesthetized using Ketamine-Xylazine. After that, the back was cleaned with 70% ethanol, then heat was stimulated using an inducer (iron metal) with a diameter of 1.0 cm which had been heated for 10 minutes, and a burn was made on the skin of the rat's back for 3 seconds (20).
Burns Healing Activity Test
To apply the test substances to the burn wounds, an ointment without the active substance was administered to the first burn wound as a negative control. On the second burn wound, a commercially available comparison product served as a positive control. The third burn wound received a test substance with an active substance concentration of 20% (ALO-20), while the fourth burn wound was treated with a test substance featuring an active substance concentration of 30% (ALO-30). The ointment was applied twice daily, and the burn wounds were subsequently covered with sterile gauze and secured with plaster. The wounds underwent daily observation for a duration of 14 days, during which they were cleaned, the dressings were changed, and applications of ointments without the active substance (negative control), the comparison product from the market, ALO-20 preparation, and ALO-30 preparation were administered.
Burns Healing Obeservation
Macroscopic observations were conducted from day 0 to day 14 to assess changes in burn wound diameter and scab formation. The measurement of burn wound diameter involved using a caliper with the Morton method, which regularly measured four directional wound diameters (see Figure 1). Subsequently, the average value of the measured diameters was calculated.
Figure 1. Four directional measurement of wound diameter.
Data Analysis
Wound diameter was analyzed using data processing SPPS software version 22 for windows program. The mean and standard deviation were calculated from each group in which there are six rats per group. Analysis of wound diameter measurement data was carried out from day 0 to day 14. The data obtained was analyzed statistically using the one-way ANOVA statistical analysis of group parameters with a significance level of 95% (p<0.05).
Result
The results of the phytochemical screening indicated that agarwood leaves tested positive for alkaloids, flavonoids, and triterpenoids, suggesting their potential use as wound healers (see Table 2).
Table 2. The phytochemical content test result of agarwood leaves.
|
Test |
Result |
|
Alkaloids identification |
++ |
|
Steroids and triterpenoids identification |
+ |
|
Flavonoids identification |
+ |
|
Saponin identification |
- |
|
Quinon identification |
++ |
|
Tannins identification |
+ |
|
Coumarin identification |
+ |
|
Essential Oil identification |
+ |
The outcomes of the nonspecific characteristics encompassed the assessment of total ash content, acid-insoluble ash, water content, loss on drying, water-soluble, and ethanol-soluble compound content. Table 3 presents the results, demonstrating the fulfillment of all non-specific characteristics.
The assessment of the placebo, positive control, ALO-20, and ALO-30 ointments, including organoleptic aspects such as physical appearance, color, and odor, homogeneity, pH, and spreadability, is presented in Table 4. The results indicate that all the ointments exhibited good spreadability, were homogeneous, and showed no signs of phase separation.
Table 3. The non-specific characteristics result of water exctract of agarwood leaf.
|
Test |
Result (%) |
|
Total ash content |
0.99 |
|
Acid-insoluble ash |
0.08 |
|
Water content |
5.95 |
|
Loss on drying |
7.02 |
|
Water-soluble compound content |
87.35 |
|
Ethanol-soluble compound content |
4.77 |
Table 4. Agarwood leaves extract ointment evaluation.
|
Formula |
Organoleptic |
Homogenity |
pH |
Spreadibility (mm) |
|
|
Color |
Odor |
||||
|
Placebo |
White |
No |
Homogen |
5.23 |
45.83 |
|
Positive control |
Dark brown |
Iodin |
Homogen |
5.35 |
61.26 |
|
ALO-20 |
Light brown |
agarwood |
Homogen |
4.24 |
36.23 |
|
ALO-30 |
Light brown |
agarwood |
Homogen |
4.33 |
33.04 |
Based on the information presented in Table 5, it is evident that the reduction achieved in ALO-30, administered with a 30% concentration of dried Four Directional Measurement of Wound Diameter agarwood leaves extract ointment, was more effective in reducing the diameter of burns compared to the negative control and ALO-20 with a concentration of 20%.
Table 5. Burn diameter by groups and days during wound healing.
|
Days |
Placebo (cm) |
Positive Control (cm) |
ALO-20 (cm) |
ALO-30 (cm) |
|
1 |
1.000±0.00 |
1.000±0.00 |
1.000±0.00 |
1.000±0.00 |
|
3 |
1.133±0.03 |
1.213±0.04 |
1.254±0.11 |
1.271±0.12 |
|
7 |
0.958±0.07 |
1.125±0.05 |
1.263±0.08 |
1.321±0.06 |
|
14 |
0.563±0.11 |
0.688±0.11 |
0.667±0.11 |
0.608±0.04 |
Notes: significant difference (p<0,05) compared to placebo
The formation of scabs is a crucial phase in the wound healing process, signifying the regeneration of skin cells to replace damaged ones. Skin cells exhibit a relatively high regeneration capacity. Throughout the 14-day observation period, daily changes were observed in the burn wounds on the rat's back, with the wound surface progressively covered by blood clotting and the development of a scab layer. The findings indicate that ALO-30 initiates scab formation on day four, whereas ALO-20 achieves this on day five, as illustrated in Table 6.
Table 6. Scab formation by groups and days in the wound healing
|
Groups |
Scab formation |
||||||||||||||
|
Days |
|||||||||||||||
|
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
|
|
Placebo |
- |
- |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
|
Positive control |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
- |
- |
- |
- |
|
ALO-20 |
- |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
- |
|
ALO-30 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
- |
- |
- |
Figure 3. Scab formation in IIA-degree burns on rats in 14 days. Scab formation in the positive treatment group occurred on day 3, in the ALO-20 on days 4, ALO-30 in days 5, and in the negative control group, new scabs formed on day 6. (A) day 0, (B) day 1, (C) day 3, (D) day 7, (E) and day 14. (1) negative control (ointment base), (2) positive control (Betadine®), (3) ALO-20, (4) ALO-30.
Discussion
In this investigation, phytochemical screening was conducted to ascertain the compound composition in the simplicia of agarwood leaves (A. malaccensis) and the water extract of agarwood leaves, which had been subjected to drying and converted into powder using a Spray Dryer. The phytochemical screening performed on the simplicia of agarwood leaves (A. malaccensis) confirmed the presence of alkaloids, flavonoids, saponins, tannins, quinones, steroids/triterpenoids, coumarins, and essential oils. Additionally, the phytochemical screening of the water extract obtained from the dried powder of agarwood leaves through spray drying revealed the presence of alkaloids, flavonoids, quinones, steroids/triterpenoids, tannins, and coumarins. The outcomes of the phytochemical screening and identification of chemical compounds in the simplicia and water extract of agarwood leaves, which underwent drying into powder using a Spray Dryer, are presented in Table 2.
In Table 3, the levels of loss on drying were measured for the two containers to determine an average drying loss level of 7.02%. The obtained results for the average loss on drying level in the spray-dried water extract of agarwood leaves met the criteria, being below 10% (16). Another specific parameter is the content of dissolved compounds, which is further categorized into ethanol-soluble and water-soluble compound contents. For the ethanol-soluble concentration test, the simplicia/extract was subjected to extraction with ethanol solvent, and the resulting juice was evaporated. The filtrat was then determined gravimetrically by repeated weighing until a constant weight was achieved. This test assesses the number of substances in the extract that dissolve in ethanol, revealing the presence of various organic compounds, both polar and non-polar. The calculation of ethanol-soluble content was performed for both glass containers, yielding an average ethanol-soluble essence content of 4.77%.
In the water-soluble compound content test, the fundamental procedure involves extracting the simplicia/extract with a water solvent. Subsequently, the obtained juice was evaporated, and the dry juice was subjected to gravimetric analysis through repeated weighings until a constant weight was achieved. This test assesses the number of substances in the extract that dissolve in water, revealing the presence of organic (polar) compounds. The water-soluble essence content was computed for both glass containers, resulting in an average water-soluble essence content of 87.35. The average proportion of organic compounds in the dry extract of agarwood leaves soluble in ethanol solvent was 4.77%, while 87.35% was soluble in water solvent. The findings indicate that compounds from agarwood leaves exhibit higher solubility in water than in ethanol. This implies that polar compounds within agarwood leaves are more abundant than non-polar compounds (17). Following the determination of weighing results, the calculation of total ash content was conducted for the two porcelain crucibles, resulting in an average total ash content of 0.99%. The outcomes met the quality requirements for the extract, specifically ≤10%. The percentage of total ash content signifies the mineral content within the extract, where higher total ash content corresponds to elevated mineral levels.
In this investigation, the formulation adopted the ointment form to maximize skin contact duration. The concoction involved agarwood leaf extract serving as the active ingredient, while adeps lanae, hard paraffin, cetostearyl alcohol, and vaseline comprised the ointment base. Visual inspection revealed distinct color variations among the agarwood leaves extract ointments. The placebo ointment exhibited a white color, indicative of containing only the ointment base. Conversely, the ALO-20 and ALO-30 ointments manifested a light brown hue owing to the presence of agarwood leaves extract. Notably, each ointment variant emitted a distinct odor; the placebo was odorless, whereas both ALO-20 and ALO-30 exuded the characteristic aroma of agarwood extract.
Homogeneity testing demonstrated consistent outcomes for the placebo, ALO-20, and ALO-30 ointments, signifying uniform mixing of all ingredients within the base. This ensured a proper integration of agarwood leaves extract with ALO-20 and ALO-30. Subsequently, the acid-insoluble ash content in the porcelain crucibles was computed, resulting in an average acid-insoluble ash content of 0.08%. This met the quality criteria for acid-soluble ash content, which stipulates a threshold of ≤1%. The acid-insoluble ash content indicates the presence of mineral or metal contaminants resistant to acid dissolution. The percentage of acid-insoluble ash content also provides insights into the existence of silicate content from soil or sand, along with metallic elements such as silver, lead, and mercury, which collectively amounted to 0.08%.
The formulation of the ointment adhered to the optimal pH range for normal skin, specifically 4.1-5.8 (21). Upon assessing the pH levels at room temperature (25-30°C), it was determined that the pH of the ointment base measured 5.23, the comparison product ointment displayed a pH of 5.35, ALO-20 recorded a pH of 4.24, and ALO-30 exhibited a pH of 4.33. These pH values comply with the stipulated standards, ensuring skin-friendliness without causing irritation.
Spreadability assessments revealed that the ointment base preparation, devoid of active substances, exhibited a spreadability of 1648.81 mm2 with a diameter of 45.83 mm. This classifies the ointment base as belonging to the semi-rigid ointment category. In contrast, the comparison product preparation demonstrated a spreadability of 2945.94 mm2 with a diameter of 61.26 mm, categorizing it as a semi-liquid ointment. ALO-20 displayed a spreadability of 1030.40 mm2 with a diameter of 36.23 mm, placing it in the semi-rigid ointment category. ALO-30 recorded a spreadability of 856.94 mm2 with a diameter of 33.04 mm, similarly classified as a semi-rigid ointment. Both formulations met the standard requirement for good ointment spreadability, falling within the range of 5–7 cm. This ensures effective dispersion, facilitating broader contact between the active substance and the burn-affected skin. Consequently, the ease of application and adherence to the affected skin regions are enhanced (22).
Agarwood leaves extract has been verified to contain alkaloid compounds with antimicrobial properties (23) and flavonoid compounds that aid in the wound healing process while also serving as antioxidants (18, 24). Flavonoids play a crucial role as antimicrobials, stimulating the growth of new cells (25). The scab formation serves the purpose of maintaining wound cleanliness, shielding the underlying tissue, preventing oxidative effects on the wound, and inhibiting potential infections by microorganisms or bacteria surrounding the wound, thereby promoting effective wound healing. Notably, scabs in the ALO-20 group took longer to form compared to those in ALO-30 and the positive control.
The positive control group utilized Betadine® ointment, which contains 10% povidone iodine. The components in Betadine® ointment act as antiseptics and contribute to expediting the wound healing process. Rapid scab formation serves as a protective barrier, segregating the external and internal areas of the wound, thereby accelerating the wound healing process. Healing times for burn wounds differed, with burns in the positive control group exhibiting the quickest recovery, followed by burns in the ALO-30 group, the ALO-20 group, and, lastly, the negative control group (see Figure 2). Various uncontrollable factors, such as wound depth, the experimental animals' resistance, and the animals' excessive activity, influenced the healing time for burn wounds. Researchers sought to minimize excessive activity by tending to closed wounds and reducing the number of experimental animals in each cage.
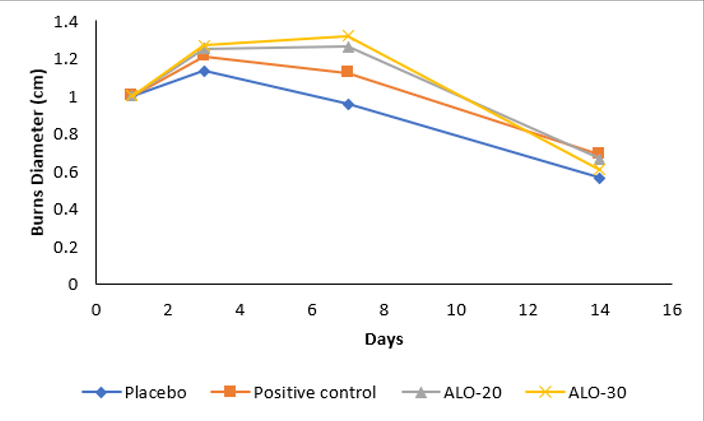
Figure 2. Burns diameter of rats during 14 days of treatment.
The scab's formation signifies the initial phase of proliferation in the wound healing process. The proliferative stage is characterized by the development of granulations within the wound, involving fibroblasts and inflammatory cells. This stage spans from day 4 to day 14. The scab, present on the wound's surface, aids in hemostasis and prevents contamination by microorganisms. Scab formation results from the denaturation of skin layer proteins in the coagulation zone (24). These processes necessitate a series of interconnected steps to safeguard the blood vessel and establish a matrix facilitating the infiltration of cells required in subsequent healing phases (11). The coagulation cascade is triggered through both extrinsic and intrinsic pathways, initiating platelet aggregation and clot formation (11).
On the initial day of injury assessment, the examination revealed an inflammatory response leading to an enlargement of the wound. To safeguard the blood vessels and establish a matrix allowing cell invasion in later healing stages, these processes involve a series of interconnected steps. The coagulation cascade, activated by extrinsic and intrinsic pathways, induces blood clotting through thrombin and fibrin, along with platelet aggregation and clot formation due to the contraction of smooth muscle walls in damaged blood arteries (26).
Soft, reddish granulation tissue is formed at the conclusion of the inflammatory phase, contributing to wound healing. Macrophages, serving as vital regulatory cells storing potent tissue growth factors, play a crucial role in later inflammatory response stages (27). Following the injury, platelets and inflammatory cells release growth factors like platelet-derived growth factor (PDGF) and transforming growth factor (TGF-), attracting fibroblasts and myofibroblasts for proliferation in surrounding tissue for three days before migrating into the wound. The subsequent stage is the proliferative phase, characterized by an active fibroblast phase moving into the surrounding tissue and forming collagen, serving as granulation tissue for wound repair (28). Neovascularization, the process of creating new blood vessels, occurs during fibroblastic proliferation and is crucial for wound healing. Collagen-producing fibroblasts proliferate once blood vessels are established. Collagen, a triple-chain glycoprotein, is abundantly produced by fibroblasts and aids in strengthening scar tissue. Fibroblasts initiated collagen production on day four after the injury, accelerating in the first three weeks and persisting for two to four weeks. Myofibroblasts, altered fibroblasts, facilitate tissue contraction to assist in forming new tissue, sealing the wound (28, 29).
From day 0 onward, the burn wound diameter initially expanded and subsequently decreased on the following day. This phenomenon is attributed to heat-induced cellular damage, leading to cell clumping and the release of inflammatory mediators, marking the initiation of the wound healing process. Macroscopic observations over 14 days revealed the average wound diameter on day 14 in the ALO-20 group was 0.667 cm, for the ALO-30 group it was 0.608 cm, for the negative control group it was 0.688 cm, and for the positive control group, it was 0.563 cm.
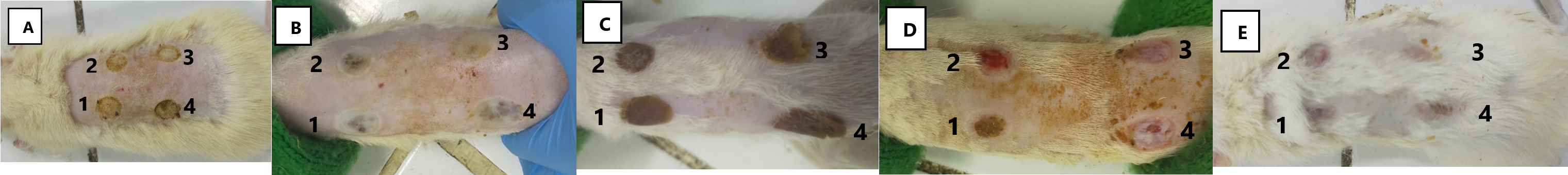
Figure 3. Scab formation in IIA-degree burns on rats in 14 days. Scab formation in the positive treatment group occurred on day 3, in the ALO-20 on days 4, ALO-30 in days 5, and in the negative control group, new scabs formed on day 6. (A) day 0, (B) day 1, (C) day 3, (D) day 7, (E) and day 14. (1) negative control (ointment base), (2) positive control (Betadine®), (3) ALO-20, (4) ALO-30.
Analyzing the 14-day observations, an evident impact of administering agarwood leaves extract ointment on reducing the diameter of grade IIA burns was noted. Statistical tests yielded a p-value of 0.031, indicating significance (p < 0.05) and revealing a substantial difference in the burn wound healing process concerning wound diameter. Subsequent Post Hoc Test results demonstrated a significant difference between formula 1 and formula 2 compared to the positive control.
Scabs formed in each treatment were notably hard and stiff. In the negative control group, with an ointment base lacking active ingredients, the average scab formation occurred on day 6. The positive control group, utilizing a market comparison product, witnessed scab formation on average by day 3. The ALO-20 group showed scab formation on the 5th day, while the ALO-30 group exhibited scab formation by the 4th day. The brownish color of scabs in the ALO-20 and ALO-30 groups resulted from the light brown hue of aloe leaf extract contained in both formulas. Observable differences in scab formation days existed among the negative control, positive control, ALO-20, and ALO-30 groups. The negative control group, lacking active substances, exhibited a comparatively longer time for scab formation, relying solely on the body's immune response to burns. ALO-20 and ALO-30 groups displayed faster average scab formation, attributed to the presence of agarwood leaves extract, aiding in expediting the wound healing process. Wound healing was complete within 14 days.
In burns treated with ALO-20 and ALO-30, scab formation was expedited, attributed to the compounds present in Agarwood leaves. The formed scab plays a crucial role in maintaining wound cleanliness and preventing microbial intrusion. This protective scab acts as a barrier, segregating the external and internal regions of the wound. Prompt scab formation accelerates the wound healing process, leading to a faster recovery (30). The compounds responsible for this effect include alkaloids, flavonoids, and triterpenoids. Alkaloids contribute to collagen fibril reinforcement by hindering cell damage through DNA synthesis. The maturation of collagen tissue in the wound area triggers hydroxyproline enzyme synthesis, and alkaloids act as potent astringents and antimicrobials, facilitating the re-epithelialization process in wounded tissue and increasing the weight of dry granulation tissue (22).
Another identified metabolite in agarwood leaf extract is quercetin, exhibiting anti-inflammatory effects by inhibiting the cyclooxygenase (COX) enzyme, which is pivotal in the formation of prostaglandins as inflammatory mediators. Additionally, antibacterial activity hampers bacterial growth by impeding bacterial hydrolytic enzymes (31). Flavonoids, another crucial compound in agarwood, contribute to wound healing by diminishing inflammatory mediators NO and PGE2, enhancing collagen expression, activating Akt signaling pathways, modifying inflammatory cytokines and growth factors, and promoting keratinocyte differentiation and motility (11). Triterpenoids also exhibit wound healing properties, particularly anti-inflammatory activity demonstrated by inhibiting NO production by RAW264.7 (32).
Daily treatment administered to the injured rats' backs in this study resulted in a gradual reduction in wound diameter and scab formation. This prevented wound oxidation and safeguarded against bacterial and foreign object contamination, thereby expediting the wound healing process. The increased presence of fibroblasts in the wound site contributes to enhanced collagen production, resulting in thicker collagen and accelerated healing. The study indicates that agarwood leaf extract in F II ointment at a 30% concentration may positively influence burn healing. As such, this natural ingredient warrants further investigation, particularly in isolating its active components.
Conclusion
Agarwood (Aquilaria malaccensis) leaves extract contains alkaloids, flavonoids, quinones, steroids/triterpenoids, tannins, and coumarins. Non-specific parameters of agarwood leaves extract showed a drying loss level of 7.02%, with ethanol and water-soluble compound contents of 4.77% and 87.35%, respectively. The total ash content was 0.99%, acid-insoluble ash content was 0.08%, and water content was 5.95%. The results of the extract quality parameters meet the specified qualifications.
The physicochemical characteristics of Agarwood leaves extract ointment revealed that both ALO-20 and ALO-30 have a light brown color with an agarwood odor and a homogeneous texture. The pH of ALO-20 was 4.24, and the pH of ALO-30 was 4.33. The spreadability results for the ALO-20 preparation were 1030.40 mm² with a diameter of 36.23 mm, and for the ALO-30 preparation, they were 856.94 mm² with a diameter of 33.04 mm. Hence, both ALO-20 and ALO-30 preparations can be classified as semi-rigid ointments.
A noticeable difference in scab formation days was observed among the negative control, ALO-20, and ALO-30 groups. Both ALO-20 and ALO-30 groups exhibited faster scab formation compared to the negative control, attributed to the presence of agarwood leaves extract, which aids in expediting the wound healing process due to the compounds contained in Agarwood leaves. Agarwood leaves extract ointment with concentrations of 20% and 30% demonstrated the ability to accelerate the healing process of grade IIA burns by promoting scab formation and reducing the burn diameter. Notably, the ALO-30 preparation exhibited a more pronounced effect than ALO-20.
Declarations
Acknowledgment
We thank the Phytochemistry Laboratory, Pharmacology Laboratory, Pharmaceutical Technology Formulation of Semisolida Laboratory, Faculty of Pharmacy, University of Pancasila for kindly supporting the technical issues in this study.
Ethics Statement
The research has received ethical approval from the Ethic Committee of the Faculty of Medicine, Universitas Indonesia with letter number of KET-633/UN2.F1/ETIK/PMM.00.02/2021.
Data Availability
The unpublished data is available upon request to the corresponding author.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Abraham JP, Plourde BD, Valle LJ, Nelson-Cheeseman BB, Stark JR, Sparrow EM, et al. Skin Burns . In: Shrivastava D, editor. : Theory and Application of Heat Transfer in Human. 2018. p. 723–9.
- Abazari M, Ghaffari A, Rashidzadeh H, Badeleh SM, Maleki Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Vol. 21, International Journal of Lower Extremity Wounds. SAGE Publications Inc.; 2022. p. 18–30.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiological reviews. 2019 Jan 1;99(1):665-706.
- Manzoor S, Khan FA, Muhammad S, Qayyum R, Muhammad I, Nazir U, Bashir MM. Comparative study of conventional and topical heparin treatment in second degree burn patients for burn analgesia and wound healing. Burns. 2019 Mar 1;45(2):379-86.
- Bigliardi PL, Alsagoff SA, El-Kafrawi HY, Pyon JK, Wa CT, Villa MA. Povidone iodine in wound healing: A review of current concepts and practices. International Journal of Surgery. 2017 Aug 1;44:260-8.
- Dhandge PD, Deshmukh SP. A review on role of herbal medicine in daily life. GSC Biological and Pharmaceutical Sciences. 2023;25(3):179-88.
- Hassanin K, Abdel-Wahab A, Mahmoud AA, Hashim A, Abdel-Badeea WI. Antioxidant Effects of Syzygium cumini Fruit Pulp Extract Against Cadmium-Induced Reproductive Toxicity. Journal of Veterinary Medical Research. 2024 Jan 3.
- Tyler VE. Herbal medicine: From the past to the future. Vol. 3, Public Health Nutrition. CAB International; 2000. p. 447–52.
- Dan MM, Sarmah P, Vana DR, Dattatreya A. Wound healing: concepts and updates in herbal medicine. Int J Med Res Health Sci. 2018 Jan 1;7(1):170-81
- Wang S, Yu Z, Wang C, Wu C, Guo P, Wei J. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules. 2018 Feb 7;23(2):342.
- Vitale S, Colanero S, Placidi M, Di Emidio G, Tatone C, Amicarelli F, D’Alessandro AM. Phytochemistry and biological activity of medicinal plants in wound healing: an overview of current research. Molecules. 2022 Jun 1;27(11):3566.
- Wangiyana IG. Medicinal Usage of Agarwood Resin in Form of Essential Oil: A Review. Jurnal Silva Samalas. 2019 Dec 29;2(2):86-90.
- Hashim YZHY, Kerr PG, Abbas P, Salleh HM. Aquilaria spp. (agarwood) as source of health beneficial compunds: A review of traditional use, phytochemistry and pharmacology. J Ethnopharmacol. 2016;331–60.
- Suhardiman A. Pengaruh Tempat Tumbuh Tanaman Daun Gaharu (Aquilaria malaccensis Lam) dari Dua Daerah yang Berbeda terhadap Aktivitas Antioksidan. Jurnal Kartika Kimia. 2023 May 31;6(1).
- Farnsworth NR. Biological and Phytochemical Screening of Plants. J Pharm Sci. 1966 Mar;55:225–76.
- FARMAKOPE HERBAL INDONESIA EDISI II 2017 KEMENTERIAN KESEHATAN REPUBLIK INDONESIA 615.1 Ind f.
- Departemen Kesehatan RI. Materi Medika Indonesia Jilid IV. 1980.
- Suhardiman A, Juanda D. Pengembangan Obat Herbal Fraksi Daun Gaharu (Aquilaria Malaccensis Lam) Dalam Bentuk Gel Untuk Penyembuhan Luka Bakar. Jurnal Sains dan Teknologi Farmasi Indonesia. 2019 Apr;8:16-26.
- Ghosh D, Mondal S, Ramakrishna K. A topical ointment formulation containing leaves extract of Aegialitis rotundifolia Roxb., accelerates excision, incision and burn wound healing in rats. Wound Medicine. 2019 Sep 1;26(1):100168.
- Sandhiutami NMD, Fahleni F, Miftahurrohmah N, Widhiyasari NKA, Azalia A, Amalia I. Enhanced wound healing effect of Areca catechu L. ointment via antibacterial activity and anti-inflammatory process at grade IIA burns in rats. Journal of HerbMed Pharmacology. 2023 Jun 1;12(3):388–98.
- Proksch E. pH in nature, humans and skin. The Journal of dermatology. 2018 Sep;45(9):1044-52.
- Mangga ME, NINGSIH DR, PURWATI Z, Akanadewi F. Formulation and evaluation of natural anti Candida albicans ointment containing mango leaf (Mangifera indica L.) extract. Sains Malaysiana. 2019;48(9):1907-12.
- Hendra H, Moeljopawiro S, Nuringtyas TR. Antioxidant and antibacterial activities of agarwood (Aquilaria malaccensis Lamk.) leaves. InAIP Conference Proceedings 2016 Jul 21 (Vol. 1755, No. 1). AIP Publishing.
- Apriani EF, Kornelia N, Amriani A. Optimizing Gel Formulations Using Carbopol 940 and Sodium Alginate Containing Andrographis paniculata Extract for Burn-Wound Healing. Jurnal Farmasi dan Ilmu Kefarmasian Indonesia Vol. 2023 Dec;10(3):300-11.
- Zulkefli N, Che Zahari CN, Sayuti NH, Kamarudin AA, Saad N, Hamezah HS, Bunawan H, Baharum SN, Mediani A, Ahmed QU, Ismail AF. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. International journal of molecular sciences. 2023 Feb 27;24(5):4607
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiological reviews. 2019 Jan 1;99(1):665-706.
- Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nature Reviews Immunology. 2015 Dec;15(12):731-44
- Badid C, Vincent M, Mcgregor B, Melin M, Hadj-Aissa A, Veysseyre C, Hartmann DJ, Desmouliere A, Laville M. Mycophenolate mofetil reduces myofibroblast infiltration and collagen III deposition in rat remnant kidney. Kidney international. 2000 Jul 1;58(1):51-61
- Visha MG, Karunagaran M. A review on wound healing. International Journal of Clinicopathological Correlation. 2019;3(2):50-9.
- Monroe DM, Hoffman M. The clotting system–a major player in wound healing. Haemophilia. 2012 Jul;18:11-6.
- Yang D, Wang T, Long M, Li P. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxidative Medicine and Cellular Longevity. 2020 Oct;2020.
- Rachpirom M, Pichayakorn W, Puttarak P. Preparation, development, and scale-up of standardized pentacyclic triterpenoid-rich extract from Centella asiatica (L.) Urb. and study of its wound healing activity. Heliyon. 2023 Jul 1;9(7).

 ETFLIN
Notification
ETFLIN
Notification






