Thermal Behavior of Polymers in Solid-State
by Maria Elvina Tresia Butar-butar ★ , Anis Yohana Chaerunisaa
Academic editor: Abd. Kakhar Umar
Sciences of Pharmacy 1(1): 7-17 (2022); https://doi.org/10.58920/sciphar01010008
This article is licensed under the Creative Commons Attribution (CC BY) 4.0 International License.
28 May 2022
16 Jun 2022
18 Jun 2022
24 Jun 2022
Abstract: A variety of potential polymers with chemical and physical stability characteristics and abundant availability lead to the rapid application of polymers in various fields. One of the crucial things that are crucial to be discussed from such polymers is the characteristic of thermal behavior. Each type of polymer such as natural and synthetic has different thermal characteristics, including Tc, Tg, Tm, and Td which can be the determining factor of polymer selection of processing and application temperature. The thermal properties will also affect molecular interactions, physical stability in manufacturing, distribution, and storage. Therefore, in this article will appoint a study on the thermal characteristics of natural and synthetic polymers, the effect of modification on the thermal properties of polymers, efforts to increase the stability of thermal, and polymer applications in the field of pharmaceutical technology.
Keywords: PolymersThermal behaviorThermal stabilityPhysical characteristics
Introduction
Polymers have been used extensively in the pharmaceutical, food, and cosmetics industries. For example, they are thickening, gelling, stabilizing, coating, viscosity, and surfactant agents (1). Those functions are supported because polymers have good chemical stability, adequate mechanical stability, high water solubility, large molecular weight, degree of polymerization, crystallinity, commercial availability, and various natural and synthetic polymers (2, 3). Generally, natural biopolymers are polysaccharides and proteins, while synthetic polymers are polyester and aliphatic synthesized (4). Considering the various potentials offered, it is appropriate that the discussion on polymers gets special attention, especially in pharmaceutical technology.
In pharmaceutical technology, especially in solid-state, polymers are widely used to increase the solubility of active pharmaceutical ingredients, increase amorphous stability, protect active pharmaceutical ingredients from environmental influences, and as a base for drug delivery (1). Considering the function of polymers widely used in solid-state, studies of the physicochemical properties of polymers, especially the thermal properties, need to be improved.
Some important points regarding the thermal properties of polymers, including Tc, Tg, Tm, and Td, will affect the physical stability of the polymer. Generally, the thermal properties are specifically analyzed by using DSC and TGA (5). Specific points at each presented peak certainly regardless of the presence of intermolecular forces, chain stiffness, crosslinking, pedant groups, plasticizers, and molecular weight based on the characteristics of the polymer (6).
Therefore, this article focuses on thermal properties and efforts to improve the stability of thermal polymers, the effect of modifications on the thermal properties of polymers, and the analysis of the phenomena that occur when polymers are applied in solid-state. This article's purpose is to present each of the thermal properties of natural and synthetic polymers to facilitate the selection of polymers according to the needs, methods, and treatments used, and storage based on each thermal polymer's properties.
Methodology
This review employed literature originating from Sciencedirect, PubMed, and Google Scholar by using the keywords' thermal properties of polymers', 'thermal behavior of polymers', 'thermal techniques in the characterization of polymers', 'natural biopolymers', and 'synthetic polymers'. The selected literature includes research on the thermal characteristics of biopolymers and synthetic polymers in a solid-state applied for use in the pharmaceutical field. We exclude review literature and literature that is not applied in the pharmacy field. A flowchart of the methodology can be seen in Figure 1.
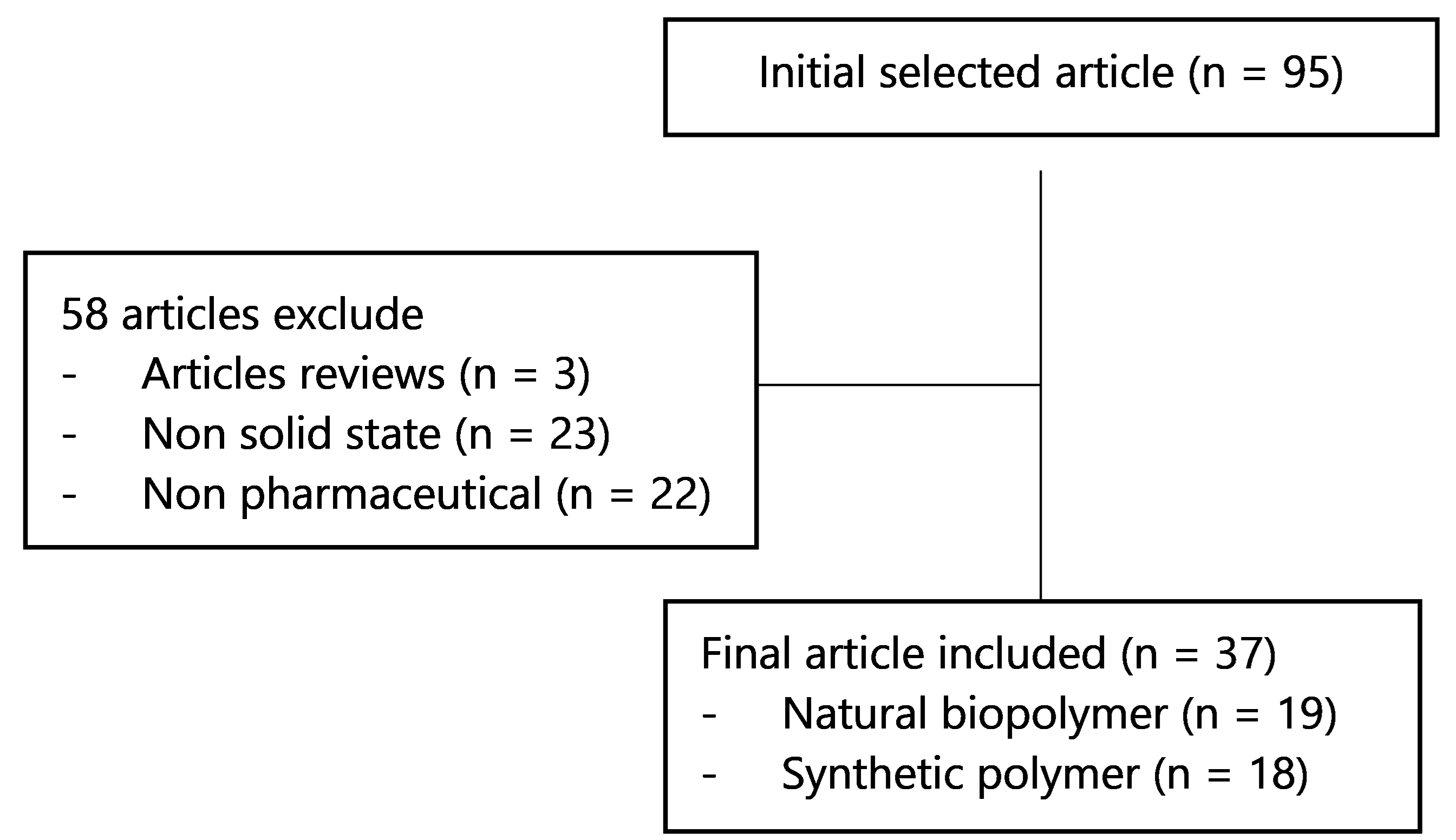
Figure 1. Flowchart of the methodology.
Discussion
Importance of Thermal Behavior Polymers
Understanding polymer thermal behavior is essential because it affects its characteristics when heat is added to or removed from a material (7). We can understand the proper storage characteristics by knowing the thermal characteristics (8). Specific thermal behavior analysis can use differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) methods (9). The analysis showed the relationship between Tg, Tm, Tc, and Td as temperature range parameters and crystallinity levels (7, 10). Thermal characteristics such as thermal equilibrium and thermodynamics, thermal capacity, and phase separation conditions are generally influenced by temperature, density, porosity, humidity, crystallinity, molecular size, and impurities (7, 11, 12). Therefore, the thermal behavior characteristics of the polymer are fundamental to obtaining the best performance when applied.
Natural Biopolymers
Biopolymers are macromolecules from microorganisms, hydrocarbons, fats, proteins, and nucleic acids (4). Many applications use natural biopolymers in the solid state, so this section will specifically discuss the thermal properties of each biopolymer presented in Table 1.
Agar
Agar is a biopolymer of the polysaccharide group. Its constituent structure consists of β-1, 3-link D-galactose, and α-1, 4-linked unit 3, 6-anhydro-L-galactose in which the side chain substituents contain sulfate ester, a group of methoxyl and pyruvate (31). However, when used, agar-based films have several critical limitations, such as thermal stability (32). Therefore, to increase agar's thermal stability, a material with a Tm / Tg higher than Tm / Tg agar needs to be added.
It is known that agar will start losing its weight at temperatures of 80 – 120 °C and 180 – 240 °C, while Td is 300 – 320 °C. This is related to the agar's volatile nature, which is easy to decompose. In the research conducted by Wang et al. (13), the addition of Bacterial cellulose (BC) to the polymer resulted in increased crystallinity, purity, and polymerization properties, excellent biodegradability, and high mechanical stability. BC could improve thermal and agar weight stability based on the results obtained. It could be seen from the results obtained at temperatures of 303.9 °C (0% BC), 308.4 °C (8% BC), and 315.6 °C (10% BC). Interactions that form hydrogen bonds between agar and BC in the form of crystals caused thermal stability to increase (33). Based on the findings, BC has the potential to be used as a thermal stability enhancer of biopolymer.
Alginate
The thermal properties of alginates which are crystalline compounds have a Tend of 74 °C due to loss of water content and Texo of 244 °C due to degradation (34). The reported results further strengthen the thermal properties, which also obtained Tend of 76 °C and Texo of 245 °C (35).
The application of alginate in pharmaceutical technology used alginate as stabilizing in a solid dispersion system (15, 16). Borba et al. (15) experimented on telmisartan (TEL) analyzed using DSC. TEL experienced an endothermic event at 265.28 °C. The incident was caused by the characteristics of the TEL that used anhydrous in the form of crystalline. In the TEL-Alginate mixture, the thermal properties changed, with endothermic events at 245 °C and exothermic events at 25 °C, which are signs of physical interaction between TEL and alginate. If only exothermic events occur, TEL is scattered in an amorphous form and forms a strong bond with alginate. Unlike the research done by Guan et al. (16) with the lovastatin drug (LOV), which also uses alginate, no significant thermal changes occurred in the mixture. Lovastatin, as a crystalline form, experiences endothermic events at a temperature of 174.5 °C, and a mixture of endothermic events occurs at a temperature of 175.4 °C. This indicates that there is no interaction between the drug and the polymer. Based on the reported above, even the same polymer will produce different thermal properties. This is influenced by crystallinity, thermal properties of active substances, drug-polymer ratio, and mixing methods.
Table 1. Thermal behavior of natural biopolymers.
Polymer | Thermal analysis | Polymer thermal behavior | Polymer characteristic | Ref. | |
Agar | DTA TGA | Td: 300 – 320 °C WL1: 80 – 120 °C WL2: 180 – 240 °C | Agar experiences two phases of degradation | (13) | |
DSC | To: 81 °C Tp: 81.9 °C Te: 82.9 °C | The higher the concentration, the higher the agar Tp will be. | (14) | ||
Alginate
| DSC | Tend: 74 °C Texo: 244 °C | Temperature affects the evaporation of water in alginate; therefore, there is decreasing in the MW of alginate. | (15) | |
DSC
| Tend: 76 °C Texo: 245 °C | Temperature influences the evaporation of water in alginate; therefore, there is decreasing in the MW of alginate. | (16) | ||
Carrageenan | DSC | Tg: 108.1 °C ΔH: 10.553 J/g | Tg is influenced by the synthesis method of carrageenan. | (17) | |
Cellulose | DSC | Tm: 48 °C | Temperature affects the elasticity of gel. | (18) | |
Chitosan | DSC | Tend: 195 – 220°C
| Chitosan oligosaccharide with MW 3900 Da has a higher Tend. | (19) | |
DTA | Tend: 175 °C | Tend is affected by the purity of chitosan, i.e.87.5%. | (20) | ||
TGA | BPEO – chitosan Td: 150 – 220 °C, WL: 20% Td: 220 – 364 °C WL: 62% | The concentration ratio of the mixture also influences the Tdeg and WL. | (21) | ||
Collagen | TGA DSC | Tend: 50 – 100 °C Texo: 250 – 350 °C | Tend and Texo were influenced by the composition of collagen. | (22) | |
Dielectric | Td Col: 75°C Col-DHT: 83 °C Col-EDC/NHS: 89 °C | Addition of DHT and EDC/NHS increases the Tdeg collagen. | (23) | ||
Gelatin | mDSC
| Tend gelatin: 35 °C | The bond transition from helix to coil induces a low Tend of gelatin. | (24) | |
mDSC | Tg gelatin 50PS: 145.2 °C | Gelatin 50PS produces higher Tg and increases thermal stability. | (25) | ||
mDSC | Tend gelatin: 40 °C Tend gelatin – genipin: 61 – 65 °C | Cross-linked increases the Tend of gelatin. | (26) | ||
Pectin | DSC | Tend: 105.64 – 113.81°C | Amorphous or semicrystalline molecule induces melting when heated. | (27) | |
DSC TGA | Td 1%: 185 °C Td 5%: 220 °C Tmax: 244 °C Residue of T 700 °C: 23.8% | The higher concentration is, also the higher Td will be. | (28) | ||
DSC TGA | Td: 210 °C WL 20% Td: 240 – 340 °C, WL 50% | Increasing temperature affects WL. The higher temperature is, also the higher % of WL will be | (29) | ||
Starch | DSC | Tm type I: 60 °C Tm type II: 90 °C | Type of amylose crystallinity influences Tm | (30) | |
Xanthan gum | DSC | To: 81.2 °C Tp: 82.3 °C Te: 83.5 °C | Concentration influences the Tp of Xanthan gum. The higher concentration is, also the higher Tp will be | (14) | |
Carrageenan
Carrageenan has been widely used because of its physicochemical properties and commercial functions. Applied κ-carrageenan to improve the thermal stability of casein (36). Based on studies reported that casein was stable at 100 °C but experienced an endothermic event around 115.8 °C (37). At the same time, the type of κ-carrageenan has a Tg of 108.1 °C with ΔH of 10.553 J/g (38). Because of the difference in thermal properties of each substance, they produced a thermal property that is in the middle of both when mixed. The increased Tg value (up to 127.2 °C) resulted from adding κ-carrageenan to casein. The ΔH value also increased to -89.22 J/g. This is caused by electrostatic interactions and the formation of hydrogen bonds between κ-carrageenan and protein domains (36). Based on this study, biopolymers not only can be used as pharmaceutical active ingredient polymers but are also able to interact with proteins, followed by changes in ΔH values.
Chitosan
The thermal properties of chitosan have Tg 203 °C and endothermic events at temperatures of 195 – 220 °C due to the degradation of saccharide rings (39). Because of it, chitosan is very suitable for protecting thermolabile or volatile APIs. Chitosan dispersed in Bunium persicum boiss. Oil (BPEO) experienced an increase in temperature degradation. Pure BPEO at a temperature of 30 – 169 °C experienced 91% weight loss, while Ne-BPEO at a temperature of 150 – 220 °C experienced a weight loss of 20 °C (21). In addition, chitosan also maintains the amorphous stability of the active substance because it can prevent crystal lattice formation from the active substance. The results of the DSC analysis indicate this. The sample was in the amorphous phase if the Tg value was obtained. However, this was influenced by comparing each substance, which also impacts the Tg value (19).
Collagen
When the collagen is given thermal influence, three helices in the collagen chain will be easily denatured. Denaturation temperature in collagen can also affect enzymatic behavior that will cause changes in physical stability (40). However, the nature of thermal collagen is also influenced by the origin of collagen. Collagen derived from marine fish has higher thermal properties by 1.7 °C than collagen originating from cultured fish (41).
When bacterial cellulose (BC) is added to collagen at temperatures of 200 °C and 400 °C, the composite loses weight by 1.4% and 25%. Even BC can reduce collagen's moisture retention from 30.5% to 26.4%. With BC, thermal collagen properties can be increased four times compared to before (22). Other efforts with the addition of dehydro thermal treatment (DHT) and 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC) in N-hydroxy-succinimide (NHS), increase the stability of collagen from 77 °C to 88 °C with crosslinked EDC/NHS and 80 °C with crosslinked DHT. This can occur due to crosslinking between collagen and water content in EDC/NHS and EDC (23). In applying collagen, you should pay attention to its origin because it dramatically affects the thermal properties of collagen.
Cellulose
Cellulose is a very hydrophilic polymer, but this property is also influenced by the crystallinity level, which ranges from 40 – 60% with a molecular weight of 127 kDa (42). The original nature of intramolecular that form hydrogen bonds also influences hydrophilicity. Therefore, many cellulose modifications have been made physically or chemically to improve cellulose deficiencies, one of which is making esters derivatives (43). Something that needs to be considered when applying cellulose as a polymer in active substances is that thermal stability depends on molecular weight because cellulose is complex; the level of cellulose used; and environmental humidity (44).
The application of cellulose in pharmaceutical technology is to increase the amorphous stability of active substances. Applied ester derivatives from cellulose, namely hydroxy propyl cellulose (HPC), increased the stability of quinine. However, the study did not report the thermal properties of HPC (44). The results obtained from HME of each quinine and quinine hydrochloride using HPC as polymers produced different Tg. The greater the concentration is used, the greater Tg will be. Besides, Tg is also influenced by the type of active substance, although it does not experience a significant difference. For example, 5% quinine with Tg of 38.11 ± 1.59 °C is different from 5% quinine hydrochloride with Tg of 39.84 ± 0.71 °C (44). These results are supported by Costanzo et al. (18), who reported the thermal properties of HPC having Tm 48 °C. It can be said that HPC is thermolabile. The thermal properties of HPC are not much different from when HPC was applied in a solid dispersion system.
Gelatin
Just like collagen, gelatin is also composed of amino acids. However, gelatin has limitations, which as having a low melting point of around 35 °C (45). At a temperature of 100 – 300 °C, gelatin has been degraded due to breaking peptide bonds on the amino acid structure (46).
Therefore, several attempts have been made to improve the stability of gelatin, such as formation with genipin as crosslinked to experience endothermic events at a temperature of 61 – 65 °C because the molecular weight increases to achieve thermodynamic balance (26). The addition of honey affects the Tm of gelatin which is more stable by increasing honey concentration. 85% honey with Tm 54.9 °C (24) and structural modification to 50PS gelatin with better physical quality has a molecular weight of 54 kDa and an isoelectric point of 8.5 with Tg of 145.2 °C (25). It is expected to obtain better gelatin stability to be applied as a polymer with the modification.
Pectin
The structure of pectin consists of three main parts (47). However, several factors will affect the physicochemical properties of the pectin, such as ionic strength, solvents, and pH (27). In addition, the temperature can affect the stability and decomposition of pectin. Pectin experienced endothermic events at 105.64 – 113.81 °C (27). Low thermal temperatures can be caused by the amorphous form of the pectin molecule. Adding other materials, such as boron nitride nanosheets (BNNSs) which are crystalline, can increase the temperature of pectin decomposition and residue % by preventing evaporation (28).
Table 2. Thermal behavior of synthetic polymers.
Polymer | Thermal analysis | Polymer thermal behavior | Polymer characteristic | Ref. |
Polyanhydrides | DSC | Tg: 83 – 106 °C | Poly(cyclic)anhydride is formed at 60 °C in two hours reaction. Polymerization using propylene oxide increases the glass transition temperature. | (53) |
TGA | Td: 286 – 331 °C | |||
Storage stability analysis | The protective antigen is stable in storage at 40 °C and -20 °C | Sebacic acid anhydride increases the stability of protective antigens both at storage temperatures of 40 °C and -20 °C. | (54) | |
TGA & DCS | Tg: 56.5 – 264.16 °C | A mixture of epoxy resin and polyanhydride produces a more stable polymer by maintaining a 21 – 48% residue at 700 °C. | (55) | |
Td:150 – 200 °C | ||||
WL: 3% | ||||
Poly(l-lactide-co-e-caprolactone) [P(LLA-CL)] | DSC | Tg: 145 – 146 °C | The physical stability (melting point) of polycaprolactone is better than polyglycolide and polylactide. | (56) |
Tm: 159.74 ± 0.64 °C | ||||
ΔH: 26.31 ± 0.58 J/g | ||||
Polyesteramides | TGA | Td: 337 – 385 °C | New aromatic polyester amides show an increase in storage modulus at temperatures of -50oC – 50 °C. The modulus storage value of this polymer differs depending on the rigidity of the meta-rings chain structure. | (57) |
DSC | WL1: 5% | |||
Td: 370 – 410 °C | ||||
WL2: 10% | ||||
Tg:190 – 220 °C | ||||
TGA | Td: 120 – 280 °C | The reaction of polyester synthesis using citric acid and mannitol occurs at temperatures of 150 °C and 170 °C. In contrast, the synthesis reaction of polyamide occurs at temperatures of 100 and 110 °C. The higher the temperature used, the faster the synthesis reaction will be. | (58) | |
DSC | WL: 43.762% | |||
Tg (polyester): | ||||
22.95 °C | ||||
Tm (polyester): 133.83 °C | ||||
| ||||
Tg (Polyamide): | ||||
5.36 °C | ||||
Tm (polyamide): 218.34 °C | ||||
DSC | Copolymers exhibit a multiphase (crystalline, amorphous physical structure) | (59) | ||
Tm: -5 – 4 °C | ||||
DSC | Tdeg: 510 °C | New polymers from amides and imides with high stability | (60) | |
WL: 5% | ||||
DMTA | Tg: 340 °C | Changes in optical rotation cause steric obstruction | (61) | |
TGA | Td: 99.7 °C | Breaking bonds between amide monomers (de-esterification) | ||
DSC | WL:10.3% | |||
Tg: -63 – 1 °C | ||||
DSC | Td: 193 °C | Changes in the melting point increase the percentage of mass loss | (64) | |
WL: 5 – 10% | ||||
Polyglycolic acid | TGA | Tm: 220 – 230 °C | PGA of low thermal stability close to the Tm | (65) |
DSC | Td: 240 °C | |||
WL: 23.3% | ||||
Polylactic acid | DSC | Td: 352.5 °C | The degradation temperature of poly (ethylene terephthalate) is decreased from 435 to 352.5 °C at 50% Polylactic Acid levels. | (66) |
TGA | Tg: 50 – 80 oC | Changes in structure from crystalline into amorphous | (67) | |
DSC | Tm :130 – 180 oC | |||
Poly(glycolide-co-lactide) (PLGA) | TGA | Td: 220 oC | Polylactides acid (PLA) and Polyglycolic acid (PLG) | (68) |
DSC | WL: 5% | |||
Poly(p-dioxanone) (PPDO) | DSC | Tg: 25 oC | PPDO thermal stability is affected by the molecular weight | (69) |
Tm: 110 oC | ||||
Td: 200 oC | ||||
MW: 3 – 4% | ||||
TGA | T5%: 240.4 oC | The carboxyl and hydroxyl groups in the PPDO are decomposed. | (70) | |
T20%: 267.2 oC | ||||
T50%: 282.3 oC | ||||
Tmax: 279.5 oC | ||||
T70%: 287.8 oC |
Starch
The second abundant and widely applied polysaccharide is starch. Even slight differences in amylopectin can affect functional properties, including thermal properties (48). Heated starch loses its water and changes into a granular structure (49). This structure plays a role in determining the crystallinity of starch. Type I starch has an amorphous form with Tm of 60 °C, while type II has a semicrystalline form with Tm of 90 °C (30). In addition, the nature of thermal starch is also influenced by its origin, such as endset starch, which experiences endothermic events at a temperature of 61.8 – 71.7 °C (50). Unlike the rice starch, the Tm occurs at 85 °C due to decreased granular crystal interactions (51). Based on this, if you want to use starch as a polymer, you should pay more attention to its origin because it heavily affects physicochemical properties.
Synthetic Polymers
Synthetic polymers are generally synthesized polyester and aliphatic (4). Generally, synthetic polymers are oxidative, resistant to hydrolytic, and have higher degradation mechanisms than natural biopolymers (52). To understand these differences, this section will discuss synthetic polymers' thermal properties, which can be seen in Table 2.
Polyanhydrides
Polyanhydrides are biodegradable surface-eroding polymers. Polyanhydrides are synthesized through dehydration from diacid molecules using the melt polycondensation method. The body can metabolize and eliminate polyanhydrides into non-toxic diacid monomers (71). The mixture of epoxy resin with bisamic acid from anhydrides produces stable polymers up to 150 – 200 °C. The degree of the stability of epoxy resins against degradation by temperature depends on the type of anhydrides used (55). Mixing cyclic anhydrides with propylene oxide produce poly (cyclic-anhydride) which has a degradation temperature above 300 °C. The more propylene oxide used, the greater the molecular weight and the higher the Tg of the formed poly (cyclic-anhydride) (53).
One of the uses of polyanhydrides in the pharmaceutical industry is to increase the stability of protective antigen proteins. Generally, proteins will be degraded in hot and cold temperatures. However, a mixture of protective antigens with polyanhydrides can survive degradation against thermal influences at 40 °C and -20 °C (54).
Polycaprolactone
Polycaprolactone (PCL), with molecular weights of 10.000 g/mol, is a polymer with suitable viscosity and rheology for excellent drug delivery (72). It is aliphatic, mostly semicrystalline shaped (73). In its application, PCL is often developed in artificial bone implants because of its good dissolution tendency, exceptional biocompatibility, and low Tm (59 – 64 °C) (74). When applied, the PCL concentration also affects the Tg. The higher the concentration, the higher the Tg. In addition to TG, the other thermal properties that can be analyzed, namely Tc, occur at 36 ° C (75). At a temperature of 56.7 °C crystallinity value is 47% (72).
Polyesteramides
Polyesteramides can be synthesized through polycondensation or ring-opening polymerization reactions using adipic and succinic acid as the basis for producing Poly (ethylene adipate) (PEA) and Poly (ethylene succinate) (PES). One of the properties of the polymers is their expansion properties in mixtures with solvents. This expanding power is influenced by the degree of crosslinking, where the higher the degree, the more swelling ability is obstructed. The increasing degree of crosslinking also correlates with the high value of modulus storage. Aromatic polyesteramide compounds have increasing modulus storage values at -50 °C up to 50 °C. The cold crystallization process might cause this. At temperatures below -100 oC, the difference in the storage value of modulus. Aromatic polyesteramide polymers are influenced by the degree of rigidity of the meta-rings structure (57). DSC studies on all mixed comparisons between polyesteramides and resins did not show a good thermogram. This might be caused by a fast curing reaction (55).
Polyglycolic Acid (PGA)
PGA is one of the earliest polymers developed for medical purposes (76). PGA rods and screws in fracture treatment do not show any side effects (77, 78). One aliphatic polymer polyester with a molecular weight of 2.000 – 42.000 g/mol with Tm 220 – 230 °C due to high crystallinity levels ranged from 86%. This will affect the thermal properties of PGA with Tg 44.8 °C (63, 79-81). Degraded at 260 – 320 °C with WL by 50% (65, 80). However, the thermal properties of PGA are also influenced by environmental conditions due to its hygroscopic nature.
Polylactic Acid (PLA)
PLA is a synthetic polymer composed of lactic acid monomers but does not contain a benzene ring is also one of the polymers of polyester aliphatic used in the biomedical (82, 83). Chemical composition and molecular structure cause the PLA's low thermal stability (84). Tg occurs at 60 °C, 124 °C, and 153 °C (85). The decrease in temperature degradation of PET is due to the shallow stability of the PLA. However, in the mixture of the two polymers, the stability of PLA increases because the degradation temperature of PET is higher (66). In addition, the addition of elastomers can improve the thermal stability of PLA (86). The mixing concentration also affects the thermal properties of PLA.
Poly(p-dioxanone) (PPDO)
PPDO is also one of the polymers of biomedical candidates (87). Molecular weight also affects this polymer's thermal, mechanical, and rheological properties. To obtain the desired thermal, the molecular weight must be controlled (88). However, low crystallinity levels lead to the shallow thermal stability of PPDO (89). The crystals contained in PPDO are formed from the melting into five spherulites different from the crystal isothermal at 60 °C. Therefore, one of the ingredients to improve the thermal stability of PPDO by adding polycarbodicide (70).
Conclusion
A natural and synthetic polymer has been widely applied in pharmaceutical technology. Both biopolymers and synthetic polymers have different thermal characteristics. Understanding the physicochemical aspects, especially factors affecting the thermal properties, namely molecular weight and origin, it is worth noting to determine the polymer's application and interaction with active pharmaceutical ingredients. Its methods and treatment, distribution, and storage maintain stability and quality.
Abbreviations
DSC, Differential scanning calorimetry; DTA, Differential thermal analysis; MW, Molecule weight; TGA, Thermogravimetric analysis; Tend, Endothermic temperature; Texo, Exothermic temperature; Tc, Crystallization temperature; Td, Degradation temperature; Te, Endset temperature; Tg, Glass transition temperature; Tm, Melting temperature; To, Onset temperature; Tp, Peak temperature; WL, Weight loss; ∆Etd, Activation energy for thermal degradation.
Declarations
Ethics Statement
Not applicable.
Data Availability
Not applicable.
Funding Information
Not applicable.
Conflict of Interest
The authors declare no conflicting interest.
References
- Karolewicz B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm J. 2016;24(5):525–36.
- Lapointe M, Barbeau B. Understanding the roles and characterizing the intrinsic properties of synthetic vs. natural polymers to improve clarification through interparticle Bridging: A review. Sep Purif Technol. 2020;231(July 2019):115893.
- Huang C, Qian X, Yang R. Thermal conductivity of polymers and polymer nanocomposites. Mater Sci Eng R Reports. 2018;132(May):1–22.
- Balaji AB, Pakalapati H, Khalid M, Walvekar R, Siddiqui H. Natural and synthetic biocompatible and biodegradable polymers. Biodegradable and Biocompatible Polymer Composites. Elsevier Ltd; 2018. 3–32 bl.
- Bischoff M, Seide G, Gries T. Analytical methods for polymers and polymer fibres. Int Polym Sci Technol. 2016;43(7):T1–8.
- Balani K, Verma V, Agarwal A, Narayan R. Physical, Thermal, and Mechanical Properties of Polymers. Biosurfaces. 2015;329–44.
- Dos Santos WN, De Sousa JA, Gregorio R. Thermal conductivity behaviour of polymers around glass transition and crystalline melting temperatures. Polym Test. 2013;32(5):987–94.
- Fuliaş A, Ledeţi I, Vlase G, Vlase T. Physico-chemical solid-state characterization of pharmaceutical pyrazolones: An unexpected thermal behaviour. J Pharm Biomed Anal. 2013;81–82:44–9.
- Rathgeber C, Miró L, Cabeza LF, Hiebler S. Measurement of enthalpy curves of phase change materials via DSC and T-History: When are both methods needed to estimate the behaviour of the bulk material in applications? Thermochim Acta. 2014;596:79–88.
- Karakus G, Kaplan Can H, Sahin Yaglioglu A. Synthesis, structural characterization, thermal behavior and cytotoxic/antiproliferative activity assessments of poly(maleic anhydride-alt-acrylic acid)/hydroxyurea polymer/drug conjugate. J Mol Struct. 2020;1210.
- Mehling H, Barreneche C, Solé A, Cabeza LF. The connection between the heat storage capability of PCM as a material property and their performance in real scale applications. J Energy Storage. 2017;13:35–9.
- Niu S, Yu H, Zhao S, Zhang X, Li X, Han K, et al. Apparent kinetic and thermodynamic calculation for thermal degradation of stearic acid and its esterification derivants through thermogravimetric analysis. Renew Energy. 2019;133:373–81.
- Wang X, Guo C, Hao W, Ullah N, Chen L, Li Z, et al. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int J Biol Macromol. 2018;118:722–30.
- Nagar M, Sharanagat VS, Kumar Y, Singh L, Mani S. Influence of xanthan and agar-agar on thermo-functional, morphological, pasting and rheological properties of elephant foot yam (Amorphophallus paeoniifolius) starch. Int J Biol Macromol. 2019;136:831–8.
- Borba PAA, Pinotti M, De Campos CEM, Pezzini BR, Stulzer HK. Sodium alginate as a potential carrier in solid dispersion formulations to enhance dissolution rate and apparent water solubility of BCS II drugs. Carbohydr Polym. 2016;137:350–9.
- Guan J, Liu Q, Zhang X, Zhang Y, Chokshi R, Wu H, et al. Alginate as a potential diphase solid dispersion carrier with enhanced drug dissolution and improved storage stability. Eur J Pharm Sci. 2018;114(December 2017):346–55.
- Zia KM, Tabasum S, Nasif M, Sultan N, Aslam N, Noreen A, et al. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int J Biol Macromol. 2017;96:282–301.
- Costanzo S, Pasquino R, Donato R, Grizzuti N. Effect of polymer concentration and thermal history on the inverse thermogelation of hydroxypropylcellulose aqueous solutions. Polymer (Guildf). 2017;132:157–63.
- Huang R, Han J, Wang R, Zhao X, Qiao H, Chen L, et al. Surfactant-free solid dispersion of BCS class IV drug in an amorphous chitosan oligosaccharide matrix for concomitant dissolution in vitro - permeability increase. Eur J Pharm Sci. 2019;130:147–55.
- Sari R, Setyawan D, Retnowati D, Pratiwi R. Development of Andrographolide Chitosan Solid Dispersion System: Physical Characterization, Solubility, And Dissolution Testing. Asian J Pharm. 2019;13(1):5–9.
- Yadav A, Kujur A, Kumar A, Singh PP, Gupta V, Prakash B. Encapsulation of Bunium persicum essential oil using chitosan nanopolymer: Preparation, characterization, antifungal assessment, and thermal stability. Int J Biol Macromol. 2019;
- Albu MG, Vuluga Z, Panaitescu DM, Vuluga DM, Cǎşǎricǎ A, Ghiurea M. Morphology and thermal stability of bacterial cellulose/collagen composites. Cent Eur J Chem. 2014;12(9):968–75.
- Marzec E, Pietrucha K. Selecting the correct scaffold model for assessing of the dielectric response of collagen-based biomaterials. Colloids Surfaces B Biointerfaces. 2018;171(May):506–13.
- Nguyen HTL, Katopo L, Pang E, Mantri N, Kasapis S. Structural variation in gelatin networks from low to high-solid systems effected by honey addition. Food Res Int. 2019;121(December 2018):319–25.
- Pas T, Vergauwen B, Van den Mooter G. Exploring the feasibility of the use of biopolymers as a carrier in the formulation of amorphous solid dispersions – Part I: Gelatin. Int J Pharm. 2018;535(1–2):47–58.
- Whitehead FA, Young SA, Kasapis S. Structural relaxation and glass transition in high-solid gelatin systems crosslinked with genipin. Int J Biol Macromol. 2019;141:867–75.
- Morales-Contreras BE, Wicker L, Rosas-Flores W, Contreras-Esquivel JC, Gallegos-Infante JA, Reyes-Jaquez D, et al. Apple pomace from variety “Blanca de Asturias” as sustainable source of pectin: Composition, rheological, and thermal properties. Lwt. 2020;117:108641.
- Yang W, Yuen ACY, Ping P, Wei RC, Hua L, Zhu Z, et al. Pectin-assisted dispersion of exfoliated boron nitride nanosheets for assembled bio-composite aerogels. Compos Part A Appl Sci Manuf. 2019;119(December 2018):196–205.
- Nova MV, Nothnagel L, Thurn M, Travassos PB, Herculano LS, Bittencourt PRS, et al. Development study of pectin/Surelease® solid microparticles for the delivery of L-alanyl-L-glutamine dipeptide. Food Hydrocoll. 2019;89(November 2018):921–32.
- Marinopoulou A, Papastergiadis E, Raphaelides SN, Kontominas MG. Structural characterization and thermal properties of amylose-fatty acid complexes prepared at different temperatures. Food Hydrocoll. 2016;58:224–34.
- Xu XQ, Su BM, Xie JS, Li RK, Yang J, Lin J, et al. Preparation of bioactive neoagaroligosaccharides through hydrolysis of Gracilaria lemaneiformis agar: A comparative study. Food Chem. 2018;240:330–7.
- Nur Hanani ZA, Roos YH, Kerry JP. Use and application of gelatin as potential biodegradable packaging materials for food products. Int J Biol Macromol. 2014;71:94–102.
- Ouyang QQ, Hu Z, Li SD, Quan WY, Wen LL, Yang ZM, et al. Thermal degradation of agar: Mechanism and toxicity of products. Food Chem. 2018;264:277–83.
- Soares JP, Santos JE, Chierice GO, Cavalheiro ETG. Thermal behavior of alginic acid and its sodium salt. Eclet Quim. 2004;29(2):57–63.
- Phang YN, Chee SY, Lee CO, Teh YL. Thermal and microbial degradation of alginate-based superabsorbent polymer. Polym Degrad Stab. 2011;96(9):1653–61.
- Tang M xue, Zhu Y dan, Li D, Adhikari B, Wang L jun. Rheological, thermal and microstructural properties of casein/κ-carrageenan mixed systems. Lwt. 2019;113:108296.
- Balakrishnan G, Silva JVC, Nicolai T, Chassenieux C, Bovay C, Buczkowski J, et al. Specific effect of calcium ions on thermal gelation of aqueous micellar casein suspensions. Colloids Surfaces B Biointerfaces. 2018;163:218–24.
- Mahmood WAK, Khan MMR, Yee TC. Effects of Reaction Temperature on the Synthesis and Thermal Properties of Carrageenan Ester Wan Ahmad Kamil Mahmood, 1 Mohammad Mizanur Rahman Khan 2* and Teow Cheng Yee 1. J Phys Sci. 2014;25(1):123–38.
- Liu X, Xia W, Jiang Q, Xu Y, Yu P. Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J Agric Food Chem. 2014;62(1):297–303.
- Leikina E, Mertts M V., Kuznetsova N, Leikin S. Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci U S A. 2002;99(3):1314–8.
- Bonnie Sun P, Hoa En C, Wen Chieh S. Molecular and thermal characteristics of acid-soluble collagen from orbicular batfish: Effects of deep-sea water culturing. Int J Food Prop. 2018;21(1):1080–90.
- Guido S. Phase Behavior of Aqueous Solutions of Hydroxypropylcellulose. Macromolecules. 1995;28(13):4530–9.
- Sharma P, Modi SR, Bansal AK. Co-processing as a tool to improve aqueous dispersibility of cellulose ethers. Drug Dev Ind Pharm. 2015;41(11):1745–58.
- Jones DS, Margetson DN, McAllister MS, Yu T, Shu L, McCoy CP, et al. Thermodynamically stable amorphous drug dispersions in amorphous hydrophilic polymers engineered by hot melt extrusion. Chem Eng Res Des. 2014;92(12):3046–54.
- Minh NC, Nguyen VH, Schwarz S, Stevens WF, Trung TS. Preparation of water soluble hydrochloric chitosan from low molecular weight chitosan in the solid state. Int J Biol Macromol. 2019;121:718–26.
- Wannaphatchaiyong S, Heng PWS, Suksaeree J, Boonme P, Pichayakorn W. Lidocaine loaded gelatin/gelatinized tapioca starch films for buccal delivery and the irritancy evaluation using chick chorioallantoic membrane. Saudi Pharm J. 2019;(xxxx).
- Ralet MC, Crépeau MJ, Buchholt HC, Thibault JF. Polyelectrolyte behaviour and calcium binding properties of sugar beet pectins differing in their degrees of methylation and acetylation. Biochem Eng J. 2003;16(2):191–201.
- Bertoft E, Annor GA, Shen X, Rumpagaporn P, Seetharaman K, Hamaker BR. Small differences in amylopectin fine structure may explain large functional differences of starch. Carbohydr Polym. 2016;140:113–21.
- Hoover R, Hughes T, Chung HJ, Liu Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res Int. 2010;43(2):399–413.
- Article R. Review Article Literature Review on Enset Starch : Physico-Chemical Properties. 2014;4(3):1–6.
- Yulianingsih R, Gohtani S. Dispersion characteristics of pregelatinized waxy rice starch and its performance as an emulsifier for oil-in-water emulsions: Effect of gelatinization temperature and starch concentration. Food Hydrocoll. 2019;95:476–86.
- Gunatillake PA, Adhikari R. Nondegradable synthetic polymers for medical devices and implants. Biosynthetic Polymers for Medical Applications. Elsevier Ltd; 2016. 33–62 bl.
- Van Zee NJ, Coates GW. Alternating copolymerization of propylene oxide with biorenewable terpene-based cyclic anhydrides: A sustainable route to aliphatic polyesters with high glass transition temperatures. Angew Chemie - Int Ed. 2015;54(9):2665–8.
- Petersen LK, Phanse Y, Wannemuehler MJ, Narasimhan B. Amphiphilic Polyanhydride Nanoparticles Stabilize Bacillus anthracis Protective Antigen. 2012;
- Patel JP, Parsania PH. Spectral and thermal study of cured tetrafunctional epoxy-ester-amide polymeric framework. Des Monomers Polym. 2014;17(5):491–500.
- Dong Y, Yong T, Liao S, Chan CK, Stevens MM, Ramakrishna S. Distinctive Degradation Behaviors of Electrospun Polyglycolide, Poly(dl-Lactide-co-Glycolide), and Poly(l-Lactide-co-e-Caprolactone) Nanofibers Cultured With/Without Porcine Smooth Muscle Cells. Tissue Eng Part A. Januarie 2010;16(1):283–98.
- Sava I. New aromatic polyesteramides: Synthesis and properties. Polimery/Polymers. 2011;56(4):263–70.
- Patil AM. Synthesis and characterization of bio-based polyester and polyamide from citric acid and mannitol. Orient J Chem. 2018;34(1):538–43.
- Rokicka J, Ukielski R. Synthesis and Properties of Multiblock Terpoly(Ester-Aliphatic- Amide) and Terpoly(Ester-Ether-Amide) Thermoplastic Elastomers with Various Chemical Compositions of Ester Block. Thermoplast Elastomers - Synth Appl. 2015;
- Kim SD, Lee B, Byun T, Chung IS, Park J, Shin I, et al. Poly(amide-imide) materials for transparent and flexible displays. Sci Adv. 2018;4(10).
- Hasegawa M, Tsujimura Y, Koseki K, Miyazaki T. Poly(ester imide)s possessing low CTE and low water absorption (II). effect of substituents. Polym J. 2008;40(1):56–67.
- Ge YP, Yuan D, Luo ZL, Wang BB. Synthesis and characterization of poly(ester amide) from renewable resources through melt polycondensation. Express Polym Lett. 2014;8(1):50–4.
- Garg P, Keul H, Klee D, Möller M. Thermal properties of poly(ester amide)s with isolated, two adjacent and three adjacent amide groups within a polyester chain. Macromol Chem Phys. 2009;210(20):1754–65.
- Basterretxea A, Gabirondo E, Sanchez-Sanchez A, Etxeberria A, Coulembier O, Mecerreyes D, et al. Synthesis and characterization of poly (ε-caprolactam-co-lactide) polyesteramides using Brønsted acid or Brønsted base organocatalyst. Eur Polym J. 2017;95(March):650–9.
- Cui A, Xue S, He M, Xin J, Chen Q. The effects on thermal stability of polyglycolic acid by adding dihydrazide metal chelators. Polym Degrad Stab. 2017;137:238–43.
- Xia X-L, Wen-tao L, Xin-ying T, Xiang-yang S, Li-na W, Su-qin H, et al. Degradation behaviors, thermostability and mechanical properties of poly (ethylene terephthalate)/polylactic acid blends. J Cent South Univ. 2014;21:1725–32.
- Rydz J, Sikorska W, Kyulavska M, Christova D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int J Mol Sci. 2015;16(1):564–96.
- Silva MF, Hechenleitner AAW, Irache JM, De Oliveira AJA, Pineda EAG. Study of thermal degradation of PLGA, PLGA nanospheres and PLGA/maghemite superparamagnetic nanospheres. Mater Res. 2015;18(6):1400–6.
- Liu C, Andjelić S, Zhou J, Xu Y, Vailhe C, Vetrecin R. Thermal stability and melt rheology of poly(p-dioxanone). J Mater Sci Mater Med. 2008;19(12):3481–7.
- Ding SD, Liu ZP, Yang T, Zheng GC, Wang YZ. Effect of polycarbodiimide on the thermal stability and crystallization of poly(p-dioxanone). J Polym Res. 2010;17(1):63–70.
- Tang X, Thankappan SK, Lee P, Fard SE, Harmon MD, Tran K, et al. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine. In: Natural and Synthetic Biomedical Polymers. Elsevier; 2014. bl 351–71.
- Invernizzi M, Turri S, Levi M, Suriano R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur Polym J. 2018;101(February):169–76.
- Dash TK, Konkimalla VB. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J Control Release. 2012;158(1):15–33.
- Perveen R, Inamuddin, Nasar A. Multiwalled carbon nanotube-based nanocomposites for artificial bone grafting. In: Applications of Nanocomposite Materials in Orthopedics. Elsevier; 2019. bl 111–26.
- Gopinath S, Adarsh NN, Radhakrishnan Nair P, Mathew S. One-way thermo-responsive shape memory polymer nanocomposite derived from polycaprolactone and polystyrene-block-polybutadiene-block-polystyrene packed with carbon nanofiber. Mater Today Commun. 2020;22:100802.
- Chu CC. Materials for absorbable and nonabsorbable surgical sutures. In: Biotextiles as Medical Implants. Elsevier; 2013. bl 275–334.
- Hirvensalo E. Fracture fixation with biodegradable rods. Forty-one cases of severe ankle fractures. Acta Orthop Scand [Internet]. Oktober 1989;60(5):601–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2557718
- Böstman O, Hirvensalo E, Mäkinen J, Rokkanen P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Joint Surg Br. Julie 1990;72(4):592–6.
- Mhiri S, Mignard N, Abid M, Prochazka F, Majeste JC, Taha M. Thermally reversible and biodegradable polyglycolic-acid-based networks. Eur Polym J. 2017;88:292–310.
- Ayyoob M, Lee DH, Kim JH, Nam SW, Kim YJ. Synthesis of poly(glycolic acids) via solution polycondensation and investigation of their thermal degradation behaviors. Fibers Polym. 2017;18(3):407–15.
- Yamane K, Sato H, Ichikawa Y, Sunagawa K, Shigaki Y. Development of an industrial production technology for high-molecular-weight polyglycolic acid. Polym J. 2014;46(11):769–75.
- Riley A. Basics of polymer chemistry for packaging materials. In: Packaging Technology [Internet]. Elsevier; 2012. bl 262–86. Available at: https://linkinghub.elsevier.com/retrieve/pii/B9781845696658500123
- Ramdhanie LI, Aubuchon SR, Boland ED, Knapp DC, Barnes CP, Simpson DG, et al. Thermal and mechanical characterization of electrospun blends of poly(lactic acid) and poly(glycolic acid). Polym J. 2006;38(11):1137–45.
- Shan XY, Jiang KY, Han J, Li JC. Thermal Stability of Polylactic Acid with Cyclophosphazene Derivative and β-cyclodextrin. Procedia Eng. 2018;211:131–6.
- Coltelli MB, Bronco S, Chinea C. The effect of free radical reactions on structure and properties of poly(lactic acid) (PLA) based blends. Polym Degrad Stab. 2010;95(3):332–41.
- Phattarateera S, Pattamaprom C. Comparative performance of functional rubbers on toughness and thermal property improvement of polylactic acid. Mater Today Commun. 2019;19(March):374–82.
- Zubitur M, Gómez MA, Cortázar M. Structural characterization and thermal decomposition of layered double hydroxide/poly(p-dioxanone) nanocomposites. Polym Degrad Stab. 2009;94(5):804–9.
- Yang KK, Wang XL, Wang YZ, Huang HX. Effects of molecular weights of poly(p-dioxanone) on its thermal, rheological and mechanical properties and in vitro degradability. Mater Chem Phys. 2004;87(1):218–21.
- Ding SD, Zheng GC, Zeng JB, Zhang L, Li YD, Wang YZ. Preparation, characterization and hydrolytic degradation of poly[p-dioxanone-(butylene succinate)] multiblockcopolymer. Eur Polym J. 2009;45(11):3043–57.

 ETFLIN
Notification
ETFLIN
Notification






